As filed with the Securities and
Exchange Commission on January 25, 2021
Registration
No. 333-249833
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
AMENDMENT
NO. 3
to
Form
S-1
REGISTRATION
STATEMENT
Under
The
Securities Act of 1933
Crown
Electrokinetics Corp.
(Exact
name of Registrant as specified in its charter)
| Delaware |
|
238150 |
|
47-5423944 |
| (State
or other jurisdiction of |
|
(Primary
Standard Industrial |
|
(IRS
Employer |
| incorporation
or organization) |
|
Classification
Code Number) |
|
Identification
No.) |
1110
NE Circle Blvd.
Corvallis,
Oregon 97330
(Address,
including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Douglas
Croxall
Chief
Executive Officer
1110
NE Circle Blvd.
Corvallis,
Oregon 97330
(800)
674-3612
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Please
send copies of all communications to:
M.
Ali Panjwani, Esq.
Pryor
Cashman LLP
7
Times Square
New
York, New York 10036
(212)
421-4100 |
|
Gregory
Sichenzia, Esq.
Sichenzia
Ross Ference LLP
1185
Avenue of the Americas, 37th Floor
New
York, New York 10036
(212)
930-9700 |
Approximate
date of commencement of proposed sale to the public:
As
soon as practicable after the effective date of this Registration Statement.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under
the Securities Act, check the following box: ☐
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the
same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list
the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list
the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”
and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large
accelerated filer |
☐ |
Accelerated
filer |
☐ |
| Non-accelerated
filer |
☐ |
Smaller
reporting company |
☒ |
| |
|
Emerging
growth company |
☒ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for
complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act.
☐
CALCULATION
OF REGISTRATION FEE
| Title of Securities to be Registered | |
Proposed
Maximum
Aggregate
Offering
Price(1) | | |
Amount
of
Registration
Fee(3) | |
| Common
stock, par value $0.0001 per share(2) | |
$ | 17,000,016.00 | | |
$ | 1,854.70 | |
| Underwriter Warrants(4) | |
| - | | |
| - | |
| Common
stock underlying Underwriter Warrants (2) (4) | |
$ | 1,700,010.00 | | |
$ | 185.47 | |
| Total | |
$ | 18,700,026.00 | | |
$ | 2,040.17 | |
| (1) |
Estimated
solely for the purpose of determining the amount of registration fee in accordance with Rule 457(o) under the Securities Act
of 1933. |
| (2) |
In
accordance with Rule 416(a), the Registrant is also registering an indeterminate number of additional shares of common stock
that shall be issuable pursuant to Rule 416 to prevent dilution resulting from share splits, share dividends or similar transaction. |
| (3) |
All
of which has already been paid. |
| (4) |
Upon
the closing of this offering, we have agreed to issue to the underwriters warrants, or
the underwriter warrants, to purchase a number of shares of common stock equal to up
to 8% of the total shares of common stock sold in the initial closing of this public
offering. The underwriter warrants will be exercisable at a per share exercise price
equal to 125% of the public offering price per share of common stock sold in this offering.
The underwriter warrants are exercisable at any time and from time to time, in whole
or in part, during the four and a half year period commencing six months
after the effective date of the registration statement related to this offering. |
The
Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until
the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become
effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall
become effective on such date as the Securities and Exchange Commission acting pursuant to said Section 8(a) may determine.
The
information in this Prospectus is not complete and may be changed. We may not sell the shares until the registration statement
filed with the Securities and Exchange Commission becomes effective. This Prospectus is not an offer to sell the shares and we
are not soliciting offers in any state where the offer or sale is not permitted.
| PRELIMINARY
PROSPECTUS |
|
SUBJECT
TO COMPLETION |
|
DATED
JANUARY 25, 2021 |
1,030,303
Shares of Common Stock
CROWN
ELECTROKINETICS CORP.
We are offering 1,030,303 common shares
pursuant to this prospectus. Our common stock is currently quoted on the OTCQB tier of the OTC Market Group, Inc. under the symbol
“CRKN.” The last reported sale price of our common stock on January 21, 2021 was $16.50 per share, as adjusted for
the one-for-three (1:3) reverse stock split of our common stock that will become effective on the date our common stock is listed
on the Exchange. At present, there is a very limited market for our common stock. We have applied to list our common stock on
The NASDAQ Capital Market (the “Exchange”) under the symbol “CRKN”. There is no assurance that our listing
application will be approved by the Exchange. If our common stock is not listed on the Exchange, we will not consummate this offering.
On October 22, 2020 our Board of Directors, and on October 24, 2020, stockholders holding a majority of our outstanding voting
shares, authorized a reverse stock split of the outstanding shares of our common stock in a range of up to one-for-six (1:6),
with our Board of Directors retaining discretion of whether to implement the reverse stock split and at which exchange ratio to
effect the reverse stock split. Although not yet effective, the Board of Directors approved a stock split ratio of one-to-three
(1:3), which reverse stock split will become effective on the date our common stock is listed on the Exchange, and all share numbers
in this prospectus have thus been adjusted to give effect to such reverse stock split, except for the financial statements and
notes thereto.
The offering is being underwritten
on a firm commitment basis. We have granted the underwriters an option to buy up to an additional 154,545 shares of common stock
from us to cover over-allotments. The underwriters may exercise this option at any time and from time to time during the 30-day
period from the date of this prospectus.
We
are an “emerging growth company”, as that term is used in the Jumpstart Our Business Startups Act of 2012, and as
such, have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings.
| | |
No Exercise of
Over-Allotment | | |
Full Exercise of
Over-Allotment | |
| | |
Per Share | | |
Total | | |
Per Share | | |
Total | |
| Public offering price | |
$ | | | |
$ | | | |
$ | | | |
$ | | |
| Underwriting discounts and commissions(1) | |
$ | | | |
$ | | | |
$ | | | |
$ | | |
| Proceeds to us, before expenses | |
$ | | | |
$ | | | |
$ | | | |
$ | | |
| (1) |
We
have also agreed to reimburse the underwriter for its expenses incurred in connection with this offering in the amount of
up to $135,000. See “Underwriting” for additional information regarding underwriter compensation. |
Investing
in our common stock involves a high degree of risks. See “Risk Factors” beginning on page 16 and elsewhere
in this prospectus for a discussion of information that should be considered in connection with an investment in our shares of
common stock.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these shares or determined
whether this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
We must raise enough to meet the Exchange’s
requirement that we have at least $15,000,000 in unrestricted publicly held shares following the closing. We will need to sell
at least 909,091 shares of common stock in order to achieve such minimum value, based on an assumed offering price of $16.50 per
common share, which was the closing price of our common stock on January 21, 2021, as adjusted for the one-for-three (1:3) reverse
stock split of our common stock that will become effective on the date our common stock is listed on the Exchange. In the event
that the offering price is lower than $16.50 per share, we will need to sell additional shares to meet the minimum value. We will
not consummate the offering unless such minimum value will be achieved.
The
underwriters expect to deliver the shares of common stock to the purchasers on or about ,
2021.
The
date of this prospectus is , 2021
| Sole
Book-Running Manager |
Lead
Manager |
| Roth Capital
Partners |
National Securities
Corporation |
| Co-Manager |
| Colliers
Securities LLC |
TABLE
OF CONTENTS
PROSPECTUS
SUMMARY
This
summary highlights information contained elsewhere in this prospectus and may not contain all of the information that you should
consider before investing in the shares. You are urged to read this prospectus in its entirety, including the information under
“Risk Factors” and our financial statements and related notes included elsewhere in this Prospectus.
On
October 22, 2020 our Board of Directors, and on October 24, 2020, stockholders holding a majority of our outstanding voting shares,
authorized a reverse stock split of the outstanding shares of our common stock in a range of up to one-for-six (1:6), with our
Board of Directors retaining discretion of whether to implement the reverse stock split and at which exchange ratio to effect
the reverse stock split. Although not yet effective, the Board of Directors approved a stock split ratio of one-to-three (1:3),
which reverse stock split will become effective on the date our common stock is listed on the Exchange. All share amounts in this
prospectus have been adjusted to give effect to this reverse stock split, except for the financial statements and notes thereto.
As
used herein, “we,” “us,” “our,” “the Company,” “Crown Electrokinetics,”
or “Crown” means Crown Electrokinetics Corporation unless otherwise indicated.
Our
Company
Overview
Crown develops and sells optical switching
film that can be embedded between sheets of glass or applied to the surface of glass, or other rigid substrates such as acrylic,
to electronically control opacity (“DynamicTint™”). Developed under an exclusive license from Hewlett-Packard
(“HP”), our technology allows a transition between clear and dark in seconds and can be applied to a wide array of
windows, including commercial buildings, automotive sunroofs, and residential skylights and windows. At the core of Crown’s
proprietary and patent-protected technology is a thin film that is powered by electrically charged pigment which not only replaces
common window tints but is also a more sustainable alternative to traditional window treatments. Crown partners with leading glass
and film manufacturers for mass production and distribution of DynamicTint.
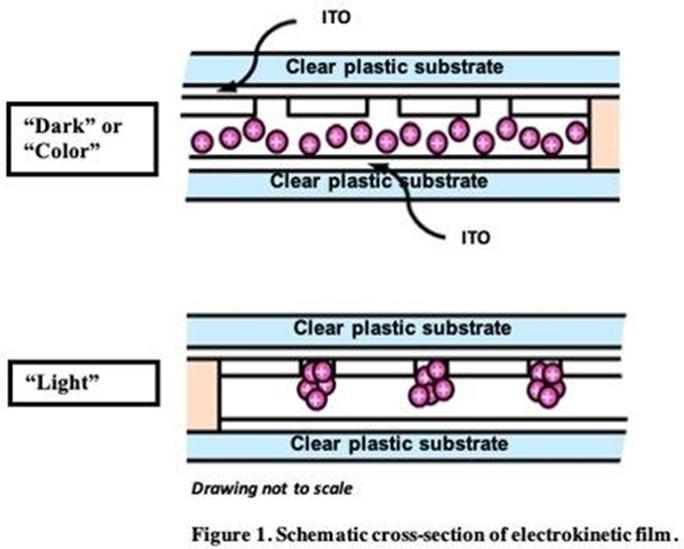
Electrokinetic
Film Technology
Crown’s
electrokinetic (EK) technology was derived from proprietary ink and microfluidic technology developed at HP. Electrokinetic refers
to the movement of particles within a fluid under the influence of an electric field. Our EK film technology utilizes nanometer-sized
pigment particles that are electrically charged and suspended in a liquid that is sandwiched between two clear substrates that
are coated with a transparent conductor oxide (TCO) film. In a non-energized state, the suspended pigment particles are distributed
uniformly between the plastic films, and will absorb, transmit, or reflect light depending on the properties of the suspended
pigment (dark state). When the proper electrical signal is applied to the conductive TCO layers, an electrical field is created
and the charged pigment particles collect in micro-embossed holes in a layer of polymer resin covering the transparent conductor
surface as shown in Figure 1. As the charged pigment particles are collected, the fluid becomes highly transparent (light
state). By applying a different electrical signal, the pigment can be dispersed back into the fluid to achieve the desired color
density or opaqueness.
Our
plastic films are manufactured using industry standard roll-to-roll (R2R) processing equipment. The Company believes its R2R processing
will have an inherently lower manufacturing cost compared to sheet-based processing methods used for other smart window technologies
like electrochromic glass. There are three basic steps to making our film using R2R equipment.
| 1) |
Deposition:
R2R TCO deposition on clear polyethylene terephthalate (PET) plastic film using vacuum sputtering of indium-tin oxide (ITO).
The ITO on PET film can be provided by a number of suppliers. Millions of square feet of ITO on PET are currently provided
for nearly all capacitance-based display touch screens. |
| 2) |
Embossing:
R2R embossing of UV-curable resin in a proprietary 3-D pattern for ink pigment control and containment on one of the two
plastic films. This process is proprietary to Crown and protected by both Crown’s patents and trade secrets. An example
of the embossed pattern is shown in Figure 2. The R2R embossing process can be completed by various plastic film
companies. Crown has the capability to accomplish the deposition and embossing steps within its current
facility. However, as production increases, one of Crown’s future manufacturing partners will handle all
production aspects of our DynamicTint film. |

Figure
2. Microscopic Optical Image of Embossed Film
| 3) |
Lamination:
The final R2R process laminates the two layers of PET together with the pigment-containing fluid (which is proprietary to
Crown and protected by patents and trade secrets) contained by the wall structure shown by the white areas in Figure 2.
The wall area has adhesion to the upper layer of PET with ITO film thereby sealing the fluid between the two plastic layers.
The fluid contains nanometer-sized pigment particles that are charged electrically and suspended in the fluid to keep the
particles evenly distributed within the film and to minimize gravitational effects due to their small size and thermal motion
of the pigment. Crown’s use of its pigment is proprietary and protected by its patents and trade secrets. |
We
believe that DynamicTintTM has the following five distinct advantages over existing optical electronic film technologies:
| |
● |
Neutral
Color – Pigment is designed to be color neutral and will not affect the hue of what is viewed through the window
in the dark or tinted state |
| |
|
|
| |
● |
Speed
– Transition time is typically under 1-2 seconds |
| |
|
|
| |
● |
Affordability
– Roll-to-Roll film manufacturing, inexpensive materials, and lower energy costs to operate DynamicTint |
| |
|
|
| |
● |
Low
Energy Requirements – Film is low voltage and can be powered by solar strip, battery, or existing electrical infrastructure |
| |
|
|
| |
● |
Retro
Fit – Film can be applied to a sheet of acrylic or heat-treated glass and attached to existing window frames, eliminating
the need to replace single pane windows with dual pane windows. |
| |
|
|
| |
● |
Sustainable
– Reduces waste, energy consumption and uses renewable energy. |
Integration
with Glass
Our
electronic film can be cut using standard laser cutting and then laminated between panes of glass for new window construction.
DynamicTint will be laminated between glass sheets for automotive applications or on a single glass sheet within an Insulated
Glass Unit (IGU) (Figure 3). An IGU typically consist of two or more panes of glass that are sealed along the edges with
an inert glass like Argon between the panes of glass to minimize heat transfer from one pane of glass to the other. Lower heat
transfer improves insulating the outside temperature from interior temperature. Power is provided to our device by two wires connected
to a single small area on each ITO surface. The wires will be routed through the IGU edge seal and can be connected to a control/power
unit attached to the IGU for individual window control. The electronics driving the film can be hardwired into the buildings HVAC
control system or controlled wirelessly depending on the customer’s needs. Because the overall power requirements are extremely
low, localized batteries in the control unit and/or in combination with a small area solar cell can be used to power DynamicTint.
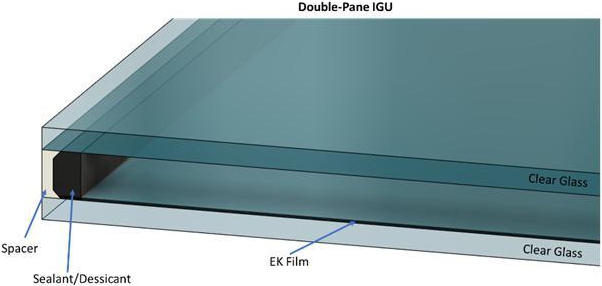
Figure
3. Double-paned IGU with EK Film
Retrofit with an Insert
DynamicTint can be laminated to other
surfaces like acrylic or heat-treated glass and the laminated sheet can be assembled in an insert (“DynamicTint Inserts”)
that can be placed over the surface of existing windows (Figure 4).
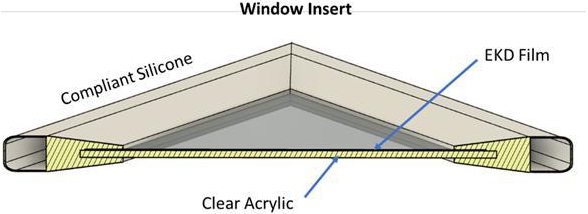
Figure
4. Window Insert with EK Film
We believe Crown’s DynamicTint
Inserts can be easily installed into residential skylights, residential windows as well as windows within garage doors. Additionally,
our DynamicTint Inserts can be used in commercial buildings to convert existing single pane windows into dual pane windows. Crown
believes that there is a significant opportunity to provide DynamicTint Inserts to commercial building owners who are looking
to eliminate window blinds, gain energy efficiency, and reduce carbon emissions.
Sustainability
Crown
is aware that working towards building a sustainable future is a common goal shared by many. Companies such as Walmart, Amazon
and Apple are now publishing their sustainability pledges, and we are seeing a trend of pledging to make their workplaces more
environmentally friendly.
Crown’s
patented technology provides a solution that helps address many sustainability issues such as:
| |
● |
Reducing
waste – as opposed to replacing single pane window units with newly manufactured dual pane windows, Crown allows
building owners to install our retrofit DynamicTint Insert into existing single pane window frames thereby creating a dual
pane window; |
| |
● |
Reducing
energy – Crown’s DynamicTint Insert when installed in a single pane window frame, can reduce energy consumption
used by HVAC’s by eliminating the need for constantly cooling and heating a room; and |
| |
● |
Using
renewable energy – Crown’s DynamicTint Insert can be powered by a solar cell that is integrated into the insert
itself thereby eliminating the need to hardwire the insert to the home or building’s electrical system. |
Crown’s
DynamicTint can reduce the amount of heat entering a building by controlling the tint of external windows. According to FacilitiesNet
(https://www.facilitiesnet.com/windowsexteriorwalls/article/Smart-Window-Benefit-Energy-Savings-Reduced-Glare--17280),
the ability to control the amount of heat entering a building reduces the heat load of the building which in turn reduces HVAC
usage. Another benefit of DynamicTint is being able to optimize daylight usage, thereby reducing the usage of lights. A study
done by Project Drawdown (https://www.drawdown.org/solutions/dynamic-glass) projected that if 30-50% of commercial building
spaces install dynamic glass, the potential climate-weighted energy efficiency from cooling is estimated at 9% and lighting at
9%—depending on local climate, building location and window orientation. This can result in 0.3-0.5 gigatons of emissions
reductions from decreased energy use.
At
Crown, we are committed to building a product that can be self-sufficient and does not require an additional power source or hard
wiring into the electrical system of a residential home or commercial building. While our DynamicTint helps keep light and heat
out of the building, we intend to harness that light and use it to power our inserts. This ensures that as we reduce a building’s
energy consumption, we are not adding to it and are working towards being carbon neutral.
Return on Investment
Crown calculates that for commercial
building owners, an average DynamicTint commercial building insert measuring five square feet would cost on average approximately
$600.00 per insert (not including installation). Using an average 500,000 square foot building with approximately 2,600 windows
that each measure five square feet, Crown estimates, based on internal research, that its insert will reduce a building owner’s
HVAC usage by up to 25% and would further eliminate the need to equip office windows with blinds. Based on the above factors,
Crown estimates energy savings of approximately $1.0 million over a ten-year period and a simple payback period of approximately
six years.
Crown further calculates that for commercial
building owners, purchasing DynamicTint as a film applied to a newly manufactured dual pane window (not including the cost of
the dual pane window) measuring five square feet would cost on average approximately $375.00 per window (not including installation).
Using an average 500,000 square foot building with approximately 2,600 windows that each measure five square feet, Crown estimates,
based on internal research, that its insert will reduce a building owner’s HVAC usage by up to 32%, or approximately $2.2
million in savings over a ten-year period and a simple payback period of approximately three years based on the same assumptions
outlined above.
Intellectual
Property
On January 31, 2016, we entered into
an IP agreement with Hewlett-Packard Development Company, L.P. and HP, Inc., collectively (“HP”), to acquire a research
license to determine the feasibility of incorporating HP’s electro-kinetic display technology in our products. Under the
terms of the agreement, the license is to be used for research purposes only, has a purchase price of $200,000 for the technology
and has a two year closing date. On April 12, 2016, the Company and HP entered into the first amendment to the agreement, which
reduced the purchase price of the technology to $175,000, of which $75,000 was payable upon completion of the technology transfer
and $100,000 was payable upon the first anniversary of the agreement’s effective date. The sales agreement entered into
with HP concurrently with the first amendment to the agreement allocated $25,000 of the $200,000 purchase price to acquire equipment
to be used in the research. On May 1, 2017, the Company and HP entered into the second amendment to the agreement which increased
the purchase price for the technology to $375,000 and extended the closing date to January 31, 2020. Of such $375,000, $75,000
is payable upon completion of the technology transfer, $100,000 is payable upon the first anniversary of the agreement’s
effective date, $100,000 is payable upon the second anniversary of the agreement’s effective date and $100,000 is payable
upon the third anniversary of the agreement’s effective date. On March 10, 2019, the Company and HP entered into the third
amendment to the agreement, which extended the closing date to January 31, 2021, enumerated certain intellectual property owned
by HP that is not subject to the exclusive license granted to the Company and revised the schedule of fees payable by the Company
to HP, such that $100,000 is payable upon the first anniversary of the agreement’s effective date, $100,000 is payable upon
the second anniversary of the agreement’s effective date and $100,000 is payable before April 20, 2019. As of January 8,
2021, the Company has paid $25,000 of the remaining $100,000 owed to HP for the research, with the balance to be paid upon the
exercise of the Company’s option to purchase the patents owned by HP by January 31, 2021. The agreement grants the Company
an option to purchase the related assignable patents at a purchase price of $1.4 million. In the event the Company exercises its
option to purchase such patents, the Company will pay to HP a running royalty in the amount of 3% of the gross revenues received
by the Company and its affiliates for the sale, rental, license or other disposition of the licensed products.
Since 2016, Crown has actively worked
to develop and license its EK technology, which it protects using patents, trade secrets and know-how. Crown currently licenses
from HP seven patents that have been issued in the United States, which the Company has the option to purchase prior to January
31, 2021 for a price of $1.4 million, subject to payment of the 3% royalty discussed above. Crown intends to exercise its option
to purchase such patents from HP on or prior to January 31, 2021. In addition, the Company has current patent applications in
the United States and other countries that if granted, would add three additional patents to its portfolio. The Company’s
United States patents expire at various dates from March 26, 2029 through September 26, 2032.
The
Company believes that its EK technology is adequately protected by its patent position and by its proprietary technological know-how.
However, the validity of the Company’s patents has never been contested in any litigation. The Company also possesses know-how
and relies on trade secrets and nondisclosure agreements to protect its technology. The Company requires any employee, consultant,
or licensee having access to its confidential information to execute an agreement whereby such person agrees to keep such information
confidential.
Company-Owned
Patents
| Application
No. |
|
Country |
|
Filing
Date |
|
Publication
No. |
|
Status |
|
Title |
| 16/259,078 |
|
USA |
|
January 28, 2019 |
|
20190256625 |
|
Pending |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| 201980018649.7 |
|
China |
|
January 28, 2019 |
|
CN111918894A |
|
Pending |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| 19704995 |
|
Europe |
|
January 28, 2019 |
|
3752867 |
|
Pending |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| 2020-566194 |
|
Japan |
|
January 28, 2019 |
|
|
|
Pending |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| 10-2020-7024977 |
|
Korea |
|
January 28, 2019 |
|
|
|
Pending |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| PCT/US2019/015464 |
|
WO |
|
January 28, 2019 |
|
WO
2019/160675 |
|
Expired |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| 62/631,623 |
|
USA |
|
February 16, 2018 |
|
|
|
Expired |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| 16/741,622 |
|
USA |
|
January 13, 2020 |
|
2020-0225552 |
|
Pending |
|
APPLICATIONS
OF AN ELECTROKINETIC DEVICE FOR AN IMAGING SYSTEM |
| PCT/US2020/013396 |
|
WO |
|
January 13, 2020 |
|
WO2020/150166 |
|
Pending |
|
APPLICATIONS
OF AN ELECTROKINETIC DEVICE FOR AN IMAGING SYSTEM |
| 62/793,250 |
|
USA |
|
January 16, 2019 |
|
|
|
Expired |
|
APPLICATIONS
OF AN ELECTROKINETIC DEVICE FOR AN IMAGING SYSTEM |
| 15/552,924* |
|
USA |
|
August 23, 2017 |
|
10,852,615 |
|
Issued |
|
TWO
PARTICLE ELECTROPHORETIC LAMINATE FOR USE WITH SMART WINDOWS WITH REDUCED DIFFRACTION |
| * | Co-owned
with University of Cincinnati |
In-Licensed
Patents
| Patent
No. |
|
Country |
|
Patent
Date |
|
Status |
|
Title |
| 8,183,757 |
|
USA |
|
May 22, 2012 |
|
Issued |
|
DISPLAY
ELEMENT |
| 8,184,357 |
|
USA |
|
May 22, 2012 |
|
Issued |
|
DISPLAY
ELEMENT |
| 8,331,014 |
|
USA |
|
December 11, 2012 |
|
Issued |
|
PIGMENT-BASED
INKS |
| 8,384,659 |
|
USA |
|
February 26, 2013 |
|
Issued |
|
DISPLAY
ELEMENT INCLUDING ELECTRODES AND A FLUID WITH COLORANT PARTICLES |
| 8,432,598 |
|
USA |
|
April 30, 2013 |
|
Issued |
|
TRANSPARENT
CONDUCTOR STRUCTURE |
| 8,896,906 |
|
USA |
|
November 25, 2014 |
|
Issued |
|
INKS
INCLUDING BLOCK COPOLYMER GRAFTED PIGMENTS VIA AZIDE CHEMISTRY |
| 8,018,642 |
|
USA |
|
September 13, 2011 |
|
Issued |
|
ELECTRO-OPTICAL
DISPLAY |
Business
Model
We
intend to develop and sell our patented EK Technology under the name DynamicTintTM. We intend to generate revenue by
selling, and in some cases licensing, DynamicTintTM film and other technical know-how to our customers and licensees
that integrate the technology into their products. We are in discussions with multiple glass manufacturers, window manufacturers,
film manufacturers, and building owners to both produce, distribute and buy DynamicTintTM film.
Applications
we are exploring with potential customers of Crown’s DynamicTint include:
| |
● |
Automotive:
sunroofs and sun visors; |
| |
● |
Aerospace,
rail and marine: windows, partitions, sun visors, and skylights; |
| |
● |
Residential
homes: residential windows, garage door windows, windows contained in and surrounding residential front doors as well as residential
skylights; and |
| |
|
|
| |
● |
Commercial
and multi-family buildings: external windows, internal glass walls and doors for both new construction and retrofit. |
Crown’s first product will be
the DynamicTint Insert for residential skylights. Crown has developed a working prototype of an insert for the residential skylight,
which allows a homeowner to control the amount of light entering the room. Crown’s patented DynamicTint Insert does not
require the homeowner to replace their skylight as it conveniently snaps into the existing frame without the need for fasteners
or any tools and can be easily installed in a matter of minutes. Usually positioned high in a home’s ceiling and out of
reach, the vast majority of existing skylights cannot prevent unwanted glare, light, or heat. Crown’s skylight insert, however,
will allow a homeowner (through a Blue Tooth connection or RF controller) to easily and quickly adjust the level of desired tint,
thereby controlling the amount of light (and subsequently heat) entering the room. The DynamicTint Skylight Insert can be powered
by either a solar cell or battery backup thereby eliminating the need to hardwire the insert to the home’s electrical system.
Utilizing the same insert strategy
as described above for existing residential skylights, Crown is in active discussions with Hudson Pacific Properties (NYSE: HPP)
(who is also a strategic investor in Crown) about deploying our DynamicTintTM film as a retrofit insert product on
their existing external glass for a majority of the 108 buildings within their portfolio. Crown’s Commercial Building Insert
would allow the building owner to quickly convert its single pane window units to a dual pane window unit. Crown’s insert
would act as the “second pane” and would allow the building owner to enjoy all the benefits of a dual pane window
without having to replace their existing single pane windows. Crown’s insert can be integrated into the building HVAC control
system, thereby optimizing the use of our DynamicTint Insert and reducing the use of the HVAC to heat or cool the rooms utilizing
our technology. As Crown’s DynamicTint technology requires very little energy to effect that transition from clear to dark
state, a solar cell coupled with a battery backup eliminates the need to hardwire the inserts to the building electrical system.
Crown believes that the potential retrofit market for its DynamicTint Building Inserts is significantly large.
Crown’s
commercialization strategies are deeply rooted in leveraging existing infrastructure. As such, Crown intends to partner with industry
leading manufacturers of glass and windows as well as manufacturers of plastic film who have roll-to-roll capabilities to efficiently
and inexpensively produce our film. While Crown will develop and own certain tooling that allows its film to be produced on a
roll-to-roll film manufacturing line, Crown will leverage the existing infrastructure of partner manufacturers to enable Crown
to avoid the extensive capital costs of building its own manufacturing facilities. In addition, Crown believes that it can also
leverage its manufacturing partner’s existing sales and distribution channels.
As
Crown moves from its research and development stage and into its first commercialization stage, it has engaged with numerous existing
and experienced thin film manufacturers about collaborating in the mass production of DynamicTint. Those ongoing discussions in
combination with the ongoing development work with two of Crown’s existing manufacturing partners, is expected to allow
Crown to move from a “development only” stage into commercialization stage in 2021.
Partners
and Customers
Crown
is in active discussions with multiple glass and film manufacturers for assessment of its DynamicTint technology and its application
to glass markets around the world. Those ongoing discussions have led to two agreements in which Crown is being paid development
revenue to provide samples and prototypes for specific markets.
On November 15, 2017, the Company entered
into a license agreement with one of the world’s leading glass manufacturers (“Glass Manufacturer”). This agreement
provides that the Company will provide samples to be used by the Glass Manufacturer for the sole purpose of determining the feasibility
of integrating the DynamicTint technology in the Glass Manufacturer’s auto and train glass products, as well as potentially
commercial and residential glass and windows. The Company began performing development activities in April of 2018. On February
1, 2019, the Company and the Glass Manufacturer entered into a new license agreement, terminating the prior agreement, which was
further extended on November 14, 2019. Under such new license agreement, the Company will provide samples to be used by the Glass
Manufacturer to evaluate the appearance of and measure optical properties of the Company’s film technology. At the Glass
Manufacturer’s option, the Company will provide additional samples to be used by the Glass Manufacturer to measure the durability
of such sample for the purpose of determining the feasibility of integrating the Company’s film technology in the Glass
Manufacturer’s auto and train glass products. The performance related to the new agreement is a continuation of the work
being performed as of April 2018. Crown has made significant improvements to its contrast ratio, haze, uniformity, switching speed,
lamination capabilities, and optical defects among other technical and visual improvements. These improvements have allowed Crown
to move from the development stage to the production/commercialization stage.
On August 23, 2017, the Company entered
into a collaborative agreement with one of the world’s leading chemical and plastic film manufacturers (“Film Manufacturer”).
The Film Manufacturer agreement provides that the Company and Film Manufacturer will jointly develop electrokinetic films and
determine their suitability for commercial use in applied films and interlayers for application to aftermarkets automobile sunroofs
and windows. The Company began performing development activities in April of 2018 and is expected to continue the relationship
and expand into commercialization of DynamicTint in 2021.
Smart
Glass Industry Trends
We
believe there are favorable converging global trends in the major near-term markets for “smart glass” products. Key
factors driving the growth of the smart glass market are the growing demand for smart glass in automobile applications, government
support through mandates and legislation for energy-efficient construction, and energy savings through smart glass applications.
In
both public and private sectors across the world, there are substantial efforts targeted toward the promotion and use of energy
efficient smart glass materials, including those used in automobiles, windows and other architectural glazings.
In
September 2020, Markets and Markets issued Smart Glass Market with COVID-19 Impact by Technology (Suspended Particle Display,
Electrochromic, Liquid Crystal), Application (Architecture, Transportation, Consumer Electronics), and Geography - Global Forecast
to 2025. The smart glass market size is expected to grow from USD 3.8 billion in 2020
to USD 6.8 billion by 2025, at a CAGR of 12.1% during the forecast period. The growth of the smart glass industry is driven by
factors, such as the growing adoption of smart glass in automotive application and, declining prices for electrochromic material.
Other major driving factors for smart glass adoption include supportive government mandates and legislation on energy
efficiency. Governing bodies of various countries are increasingly encouraging the use of these energy-efficient products. Smart
glass has inherent energy-saving and auto-dimming properties, which reduce its maintenance cost. As a result, the perceived benefits
of these glass products are more than the incurred investments.
Crown
believes that the smart glass industry is in the initial phase of growth and that DynamicTintTM may have commercial
applicability in many products where variable light-control is desired.
Our
Technology
DynamicTintTM
combines many of the favorable properties of the other smart window technologies, which are described below. It has a fast
switching time (1-2 sec.) like Suspended Particles in Polymer (SPD) and Polymer Dispersed Liquid Crystal (PDLC) technology, but
does not need alternating current power, requires less power and can tint to black. Unlike electrochromic (EC) technology, modulation
in light level is not area dependent, has much faster switching speeds, can tint to black and uses direct current pulses to change
state quickly, allowing for much lower power consumption. DynamicTint is also expected to have good bi-stability, so that when
a light level of the film is selected, the film will remain unchanged for extended time periods with little to no electrical power
required. Because of the low power requirements, DynamicTint can be powered with batteries or small area solar cells, allowing
retrofit to existing windows with ease and without the need to hard wire to existing electrical systems.
There
are also major differences resulting from the fact that different color nanoparticles can be used in DynamicTintTM
whereas competing technologies are either black, gray or blue tinted. Furthermore, with DynamicTint it is possible to use multiple
colorants in the same film, which has been demonstrated in the recent past under a research project at the University of Cincinnati.
Other
Smart Glass Technologies
Variable
light transmission technologies can be classified into two basic types: “active” technologies that can be controlled
electrically by the user either automatically or manually, and “passive” technologies that can only react to ambient
environmental conditions such as changes in lighting or temperature. Most of the technologies are “active”. One type
that is passive is thermochromic technology where a rise in temperature will darken the film applied to glass.
The
Company believes that our DynamicTint has certain performance advantages over other “smart glass” technologies and
that pricing and product performance are the two main factors critical to the adoption of smart glass products. Because the non-EK
smart glass technologies listed below do not have published, consistent pricing or cost data that can be relied upon, the Company
cannot accurately report its price position relative to these other technologies. In terms of product performance, the Company
believes that DynamicTint offers numerous advantages over other smart glass technologies as discussed below.
| Technology | |
Can
Retrofit | |
Power
Usage | |
Can
Tint to Black | |
Solar
or Battery Powered | |
Tint
Transition Speed | |
Light
Transmission |
| DynamicTintTM (Electrokinetic) | |
| |
<0.01 W/M2 | |
✓ | |
✓ | |
~2 sec | |
1.0% - 70% |
| Electrochromic (EC) | |
✕ | |
0.3 – 2 W/M2
(30X EK) | |
✕ | |
✕ | |
5-40 min | |
1% - 58% |
| Suspended Polymers in Particles (SPD) | |
✕ | |
~1.3 W/M2
(130X EK) | |
✕ | |
✕ | |
3 - 5 sec | |
3% - 62% |
| Polymer Dispersed Liquid Crystal (PDLC) | |
✕ | |
5 – 20 W/M2
(500X EK) | |
✕ | |
✕ | |
1 – 3 sec | |
~80%
* Does not block light |
Electrochromic
Glass
Electrochromic
(EC) glass technology has been used as a light absorbing technology for rear view mirrors in automobiles for decades, and more
recently for large-scale windows. However, the EC technology developed for windows is based on a different set of materials that
are directly deposited on the heat-treated glass panels. All the current EC companies are using tungsten oxide as the main component
involved in the color transition from clear to blue. Because of the nature of the chemical transition of the tungsten oxide, the
EC film does not absorb as much of the blue light and so remaining light will have a strong blue hue both in the room and looking
through the window. The speed of the switching from dark to light or the reverse change is directly related to the size of the
window area and the electrode design which brings electrical current to the EC material to start the chemical transition. EC technology
is basically a battery-like material that requires “charging and discharging”. The time to charge/discharge the EC
material in a large window can take up to 40 minutes to change form the dark state to the clear state at nominal temperatures.
Also, during switching of the EC film, there can be non-uniform areas which can vary in level of tint from center to edge. The
larger the area of the window, the more non-uniform during the change of state. Longer switching time can minimize the non-uniform
areas. The EC materials are typically vacuum deposited directly on “defect-free” glass. The typical investment required
for a large window electrochromic factory can run into the hundreds of millions of dollars, due to the large-scale vacuum equipment
required, low particulate cleanroom required, and the relatively slow speed of deposition for all the various layers. Kinestral
Technologies is using a chemical liquid deposition technique to replace some of the vacuum deposition steps to lower the capital
investment needed for manufacturing.
Suspended
Particle Glass (SPD)
SPD
is a film that has suspended long and narrow particles in an encapsulated liquid polymer film with layers of ITO on either side
to allow generation of an alternating current electrical field to twist the particles from a random state to a near vertical state
perpendicular to the ITO plane. In the vertical state light passes through the film and in the random state the light is absorbed
by the particles. The color of the film is blue since the particles used in the film don’t absorb blue light as well as
other colors of sunlight. No other types of particles have been created for this type of device. The film responds quickly to
the electrical field, however, requires constant high AC voltage to hold the clear state. The film is manufactured on plastic
and uses roll-to-roll (R2R) equipment processing. Also, because the particles are aligned when in the clear state, the film has
a limited viewing angle much like older liquid-crystal displays. When viewed at a side angle, the film will appear darker. The
current market for SPD has been mainly automobile sunroofs where the viewing angle of the passengers is relatively fixed at nearly
perpendicular angle to the SPD film.
Polymer-Dispersed
Liquid Crystal (PDLC) Film
PDLC
requires an AC electric field like the SPD film described above to achieve a clear state. However, the liquid-crystal based film
can only scatter light in the power-off state, therefore, most of the incoming light is transmitted through the film (~80%). Typically,
the PDLC film is used for interior windows or doors to create privacy. PDLC has similar manufacturing methods using R2R equipment
and plastic film with ITO conductor to the SPD film. The film is available from many Far East manufacturing companies with some
able to make ~150 cm width film. The quality of the film can vary based on the manufacturing company. The film was invented at
Kent State University in the 1980’s and the patents have expired.
Competition
Several
smart glass competitors have an operating history, including:
| |
● |
SAGE
Electrochromics, Inc., a wholly owned subsidiary of Saint-Gobain, which develops and manufactures electrochromic glass; |
| |
● |
View
Glass and Kinestral Technologies manufacture electrochromic glass at their purpose-built manufacturing facilities and both
are headquartered in California; and |
| |
● |
Research
Frontiers, Inc. licenses an electronically controlled tinted film, utilizing SPD technology, to various companies. |
Crown
Electrokinetics expects that other competitors will emerge in the future.
Research
and Development
Crown
has been using a 6” width R2R equipment capable of handling the deposition, embossing and lamination steps of the manufacturing
process for its research and development for the past three years and Crown will have its proto-manufacturing roll-to-roll equipment
at 12” width available in early 2021. Production prototypes for qualification and system testing will be sourced from the
12” equipment in 2021. Crown will utilize the 12” width film for the DynamicTint Skylight Insert. Larger scale manufacturing
is planned at a minimum of 24” width film to address markets including larger format skylights inserts, appropriately sized
residential and commercial building window inserts, and many automobile sunroofs beginning in mid-to-late 2021. Thereafter, one
of Crown’s future manufacturing partners will follow with a roll-to-roll line to accomplish all necessary steps to manufacture
DynamicTint of at least 60” width capability. This will allow Crown to address the vast majority of window sizes for all
applications.
As
a result of the Company’s research and development efforts, the Company believes that its EK technology, in some cases,
is now, or with additional development will become, usable in a number of commercial products. Such products may include one or
more of the following fields: “smart” windows, doors, skylights and partitions; self-dimmable automotive sunroofs,
windows, sun visors, and mirrors.
The
Company has devoted most of its financial resources to research and development activities with the goal of producing commercially
viable EK products and has developed working samples of its EK technology.
Crown’s
main goals in its research and development include:
| |
● |
developing
wider ranges of light transmission and quicker switching speeds, |
| |
● |
developing
different colored version of Crown’s DynamicTint, |
| |
● |
reducing
the voltage required to operate EK samples, |
| |
● |
obtaining
data and developing improved materials regarding environmental stability and longevity, and |
| |
● |
quantifying
the degree of energy savings expected by users of the Company’s technology including the degree that EK technology can
control heat and its contribution to energy savings directly and through daylight harvesting strategies in sustainable building
designs. |
Employees
The
Company has eleven full-time employees and five advisors. Seven of the employees are technical personnel, and the rest perform
business development, legal, finance, marketing, investor relations, and administrative functions. Of these employees, three have
obtained doctorates, one has a master’s degree in chemistry, and one has extensive industrial experience in electronics
and electrical engineering. Two employees also have additional postgraduate degrees in business administration, and one advisor
has a doctorate in jurisprudence. Also, the Company’s suppliers and licensees have well qualified personnel on their teams
with advanced degrees in a number of areas relevant to the commercial development of products using the Company’s technology.
The success of the Company is dependent upon, among other things, the services of its senior management, the loss of which could
have a material adverse effect upon the prospects of the Company.
As
Crown continues to grow, we will add additional engineering, marketing and executive level personnel.
Our
Corporate Information
Crown’s
Research & Development Operation currently occupies 1,700 square feet of space, located on the HP Inc. campus in Corvallis,
Oregon in the Advanced Technology and Manufacturing Institute (ATAMI). ATAMI is an academic-industrial research center and business
incubator designed to provide an advanced materials development environment to private sector partner tenants performing research
and development. The facility includes access to shared state-of-the-art tooling capabilities. ATAMI has grown to 80,000 square
feet since its inception in 2004 and now offers Crown all the space requirements it needs for the foreseeable future.
Crown
is located at 1110 NE Circle Blvd, Corvallis, OR 97330. Our telephone number is +1 (800) 674-3612 and our Internet website address
is www.crownek.com.
Crown
Electrokinetics Corp. was incorporated in the State of Delaware on April 20, 2015. Effective January 14, 2016, the Company’s
name was changed to 3D Nanocolor Corp. (“3D Nanocolor”) from 2D Nanocolor Corp. Subsequently, effective October 6,
2017, the Company’s name was changed to Crown Electrokinetics Corp. from 3D Nanocolor Corp. Prior to August 22, 2017, 3D
Nanocolor was a wholly owned subsidiary of Marathon Patent Group (“Marathon”). On August 22, 2017, Marathon entered
into a Retention Agreement with Doug Croxall, Marathon’s Chief Executive Officer and Chairman of the Board of Directors
(the “Retention Agreement”). As part of the Retention Agreement, Mr. Croxall received all of the outstanding shares
of 3D Nanocolor’s common stock held by Marathon and 1,000,000 stock warrants which had no value at the time of transfer.
On September 29, 2017, Marathon transferred to LVL Patent Group, LLC, an entity wholly-owned by Mr. Croxall, all of Marathon’s
title and interest to, and its ownership in, the common stock of 3D Nanocolor Corp.
Exchange
Listing
We
have filed an application to list our common stock on the Exchange under the symbol “CRKN”. No assurance can be given
that our application will be approved. If our application to the Exchange is not approved or we otherwise determine that we will
not be able to secure the listing of the common stock on the Exchange, we will not complete the offering.
Implications
of Being an “Emerging Growth Company”
As
a public reporting company with less than $1.07 billion in revenue during our last fiscal year, we qualify as an “emerging
growth company” under the Jumpstart Our Business Startups (JOBS) Act. An emerging growth company may take advantage of certain
reduced reporting requirements and is relieved of certain other significant requirements that are otherwise generally applicable
to public companies. In particular, as an emerging growth company we:
| |
● |
are
not required to obtain an attestation and report from our auditors on our management’s assessment of our internal control
over financial reporting pursuant to the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act; |
| |
● |
are
not required to provide a detailed narrative disclosure discussing our compensation principles, objectives and elements and
analyzing how those elements fit with our principles and objectives (commonly referred to as “compensation discussion
and analysis”); |
| |
● |
are
not required to obtain a non-binding advisory vote from our stockholders on executive compensation or golden parachute arrangements
(commonly referred to as the “say-on-pay,” “say-on-frequency” and “say-on-golden-parachute”
votes); |
| |
● |
are
exempt from certain executive compensation disclosure provisions requiring a pay-for-performance graph and CEO pay ratio disclosure; |
| |
● |
may
present only two years of audited financial statements and only two years of related Management’s Discussion & Analysis
of Financial Condition and Results of Operations, or MD&A; and |
| |
● |
are
eligible to claim longer phase-in periods for the adoption of new or revised financial accounting standards under §107
of the JOBS Act. |
We
intend to take advantage of all of these reduced reporting requirements and exemptions, including the longer phase-in periods
for the adoption of new or revised financial accounting standards under §107 of the JOBS Act. Our election to use the phase-in
periods may make it difficult to compare our financial statements to those of non-emerging growth companies and other emerging
growth companies that have opted out of the phase-in periods under §107 of the JOBS Act.
Certain
of these reduced reporting requirements and exemptions are also available to us due to the fact that we also qualify as a “smaller
reporting company” under the SEC’s rules. For instance, smaller reporting companies are not required to obtain an
auditor attestation and report regarding management’s assessment of internal control over financial reporting; are not required
to provide a compensation discussion and analysis; are not required to provide a pay-for-performance graph or CEO pay ratio disclosure;
and may present only two years of audited financial statements and related MD&A disclosure.
Under
the JOBS Act, we may take advantage of the above-described reduced reporting requirements and exemptions until December 31, 2024,
or such earlier time that we no longer meet the definition of an emerging growth company. In this regard, the JOBS Act provides
that we would cease to be an “emerging growth company” if we have more than $1.07 billion in annual revenues, have
more than $700 million in market value of our common stock held by non-affiliates, or issue more than $1 billion in principal
amount of non-convertible debt over a three-year period. Furthermore, under current Securities and Exchange Commission rules,
we will continue to qualify as a “smaller reporting company” for so long as we have (i) a public float (i.e., the
market value of common equity held by non-affiliates) of less than $250 million as of the last business day of our most recently
completed second fiscal quarter or (ii) (A) if we have no public float or a public float of less than $700 million and (B) annual
revenues of less than $100 million during our most recently completed fiscal year.
About
This Offering
| Shares of common stock
being offered: |
|
1,030,303
shares of common stock, based on an assumed offering price of $16.50 per common share (the last reported sale price of our
common stock on the OTCQB on January 21, 2021, as adjusted for the one-for-three (1:3) reverse stock split of our common stock
that will become effective on the date our common stock is listed on the Exchange). |
| |
|
|
| Number of shares of common stock outstanding
after this offering(1): |
|
9,412,780 shares
of common stock (or 9,567,325 shares if the underwriters exercise their option to purchase additional shares in full). |
| |
|
|
| Underwriters’ option to purchase additional
shares |
|
We have granted the underwriters a 30-day
option to purchase up to 154,545 additional shares of common stock at the public offering price, less underwriting discounts
and commissions, on the same terms as set forth in this prospectus. |
| |
|
|
| Gross proceeds to us, net of underwriting
discounts and commissions but before expenses: |
|
$15,640,014.70,
or $17,986,014.7 if the underwriters exercise their option to purchase additional shares in full, based on an assumed public
offering price of $16.50 per share (the last reported sale price of our common stock on the OTCQB on January 21, 2021, as
adjusted for the one-for-three (1:3) reverse stock split of our common stock that will become effective on the date our common
stock is listed on the Exchange). |
| |
|
|
| Use of Proceeds: |
|
We plan to use the
net proceeds of this offering primarily for capital expenditures, working capital and other general corporate purposes, in
addition to the purchase of seven patents that we currently license from HP. For more information on the anticipated use of
proceeds of this offering, see “Use of Proceeds” on page 23 of this prospectus. |
| Proposed
Exchange Trading Symbol: |
|
Our
common stock is currently traded on the OTCQB over the counter market under the symbol “CRKN.” We have
applied to list our common stock on the Exchange under the symbol “CRKN”. The listing of our common stock on the
Exchange is a condition of consummating this offering. |
| |
|
|
| Risk
factors: |
|
Investing
in our shares of common stock involves a high degree of risk. As an investor, you should be able to bear a complete loss of
your investment. You should carefully consider the information set forth in the “Risk Factors” section
beginning on page 16. |
| (1) |
The
number of shares of common stock outstanding immediately following this offering is based on 7,649,346 shares outstanding
as of January 11, 2021, assumes (a) the effectiveness of a 1:3 reverse stock split, (b) the conversion by a convertible noteholder
of an aggregate of $343,423.02 of debt pursuant to such notes into 360,111 shares of common stock and 251 shares of Series
A Preferred Stock simultaneously with the closing of this offering, (c) the conversion by a convertible noteholder of an aggregate
of $1,496,751.82 of debt pursuant to such notes into 173,111 shares of common stock and 1,443 shares of Series B Preferred
Stock simultaneously with the closing of this offering and (d) the conversion by other holders of the Company’s convertible
promissory notes in an aggregate principal amount of $749,660 into 199,910 shares of common stock at a conversion price of
$3.75 per share simultaneously with the closing of this offering, and excludes: |
| |
● |
7,333,333
shares of common stock reserved for issuance under our 2016 Equity Incentive Plan; |
| |
● |
5,333,333
shares of common stock reserved for issuance under our 2020 Employee Incentive Plan; |
| |
● |
10,388,978
shares of common stock issuable upon the exercise of options (of which 7,333,333 have vested) at a weighted average exercise
price of $2.64 per share as of January 11, 2021; |
| |
● |
2,207,349
shares of common stock issuable upon the conversion of 251 shares of Series A Preferred Stock and 1,443 shares of Series B
Preferred Stock; and |
| |
● |
410,453 shares
of common stock issuable upon the conversion of outstanding convertible promissory notes (not including shares of preferred
stock to be issued to certain convertible noteholders). |
In
addition, unless we specifically state otherwise, the information in this prospectus assumes:
| |
● |
a
public offering price of $16.50 per share, the last reported sale price of our common stock on the OTCQB on January 21, 2021,
as adjusted for the one-for-three (1:3) reverse stock split of our common stock that will become effective on the date our
common stock is listed on the Exchange. |
Selected
Financial Information
The
following selected income statement data for the six months ended September 30, 2020 and 2019, and for the years ended March 31,
2020 and 2019 and the selected balance sheet data as of September 30, 2020, March 31, 2020 and 2019 have been derived from
our audited financial statements included elsewhere in this prospectus. This financial data should be read in conjunction with
“Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements
and related notes included elsewhere in this prospectus. The historical results presented below are not necessarily indicative
of the results that may be expected in any future period.
| | |
Six Months Ended
September 30, | | |
Years Ended
March 31, | |
| | |
(Unaudited) | | |
| |
| | |
2020 | | |
2019 | | |
2020 | | |
2019 | |
| Selected Income Statement Data: | |
| | |
| | |
| | |
| |
| Revenue | |
$ | - | | |
$ | - | | |
$ | 100,000 | | |
$ | 504,788 | |
| Gross loss | |
$ | - | | |
$ | (307,000 | ) | |
$ | (520,000 | ) | |
$ | (109,212 | ) |
| Research and development | |
$ | (1,795,696 | ) | |
$ | (1,070,766 | ) | |
$ | (1,826,140 | ) | |
$ | (712,116 | ) |
| Selling, general and administrative | |
$ | (9,597,169 | ) | |
$ | (2,868,391 | ) | |
$ | (5,491,769 | ) | |
$ | (1,791,103 | ) |
| Loss From Operations | |
$ | (11,392,865 | ) | |
$ | (4,246,157 | ) | |
$ | (7,837,909 | ) | |
$ | (2,612,431 | ) |
| Net Loss | |
$ | (16,228,512 | ) | |
$ | (5,026,430 | ) | |
$ | (9,603,871 | ) | |
$ | (4,295,753 | ) |
| Net Loss per Common Share: | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (3.75 | ) | |
$ | (2.30 | ) | |
$ | (3.90 | ) | |
$ | (2.00 | ) |
| Diluted | |
$ | (3.75 | ) | |
$ | (2.30 | ) | |
$ | (3.90 | ) | |
$ | (2.00 | ) |
| Cash Dividends per Common Share | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | |
| | |
September 30,
2020 | | |
March 31,
2020 | | |
March 31,
2019 | |
| | |
(Unaudited) | | |
| | |
| |
| Selected Balance Sheet Data: | |
| | |
| | |
| |
| Property and equipment, net | |
$ | 106,600 | | |
$ | 92,629 | | |
$ | 102,378 | |
| Total Current Assets | |
$ | 692,626 | | |
$ | 61,000 | | |
$ | 191,113 | |
| Total Assets | |
$ | 1,014,032 | | |
$ | 388,636 | | |
$ | 673,039 | |
| Total Current Liabilities | |
$ | 7,605,204 | | |
$ | 7,349,133 | | |
$ | 4,067,682 | |
| Total Liabilities | |
$ | 7,605,204 | | |
$ | 7,349,133 | | |
$ | 4,067,682 | |
| Working Capital (deficit) | |
$ | 6,912,578 | | |
$ | 7,288,133 | | |
$ | 3,876,569 | |
| Common Stock, par value $0.0001 | |
$ | 2,864 | | |
$ | 1,733 | | |
$ | 988 | |
| Additional paid-in capital | |
$ | 26,082,835 | | |
$ | 9,486,129 | | |
$ | 3,448,857 | |
| Accumulated Deficit | |
$ | (32,676,871 | ) | |
$ | (16,448,359 | ) | |
$ | (6,844,488 | ) |
| Stockholders’ Deficit | |
$ | (6,591,172 | ) | |
$ | (6,960,497 | ) | |
$ | (3,394,643 | ) |
RISK
FACTORS
An
investment in our common stock involves a high degree of risk. The risks described below include all material risks to our company
or to investors in this offering that are known to our company. You should carefully consider such risks before participating
in this offering. If any of the following risks actually occur, our business, financial condition and results of operations could
be materially harmed. As a result, the trading price of our common stock could decline, and you might lose all or part of your
investment. When determining whether to buy our common stock, you should also refer to the other information in this prospectus,
including our financial statements and the related notes included elsewhere in this prospectus.
Risks
Relating To Our Business
In
addition to the other information in this prospectus, you should carefully consider the following factors in evaluating us and
our business. This prospectus contains, in addition to historical information, forward-looking statements that involve risks and
uncertainties, some of which are beyond our control. Should one or more of these risks and uncertainties materialize or should
underlying assumptions prove incorrect, our actual results could differ materially. Factors that could cause or contribute to
such differences include, but are not limited to, those discussed below, as well as those discussed elsewhere in this prospectus,
including the documents incorporated by reference.
There
are risks associated with investing in companies such as ours who are primarily engaged in research and development. In addition
to risks which could apply to any company or business, you should also consider the business we are in and the following:
Source
and Need for Capital.
As
we take steps in the commercialization and marketing of our technology, or respond to potential opportunities and/or adverse events,
our working capital needs may change. We anticipate that if our cash and cash equivalents are insufficient to satisfy our liquidity
requirements, we will require additional funding to sustain our ongoing operations and to continue our research and development
activities.
We
have funded most of our activities through sales of our securities to investors. Eventual success of the Company and generation
of positive cash flow will be dependent upon the extent of commercialization of products using the Company’s technology.
We can give no assurances that we will generate sufficient cash flows in the future (through sales of our common stock, exercise
of options and warrants, royalty fees, or otherwise) to satisfy our liquidity requirements or sustain future operations, or that
additional funding, if required, will be available when needed or, if available, on favorable terms.
History
of Operating Losses.
We have experienced net losses from
operations, and we may continue to incur net losses from operations in the future. We have incurred substantial costs and expenses
in researching and developing our electrokinetic technology. As of September 30, 2020, we had a cumulative net loss of $32.7
million since our inception. Our net loss was approximately $16.2 million for the six months ended September 30, 2020, and $9.6
million and $4.3 million during the years ended March 31, 2020 and 2019, respectively (which includes non-cash accounting charges
during the year ended March 31, 2020 of approximately $6.8 million and for the year ended March 31, 2019, of approximately $2.7
million, resulting from stock-based compensation expenses related to our stock options, amortization of our debt discount related
to our convertible notes, the change in fair value of our warrant liability, and depreciation and amortization).
We
expect to continue to incur losses from operations and negative cash flows, which raise substantial doubt about our ability to
continue as a Going Concern.
We
anticipate incurring additional losses until such time, if ever, that we can obtain marketing approval to sell, and then generate
significant sales, of our technology that is currently in development. Substantial additional financing will be needed by the
Company to fund our operations and to develop and commercialize our technology. These factors raise substantial doubt about the
Company’s ability to continue as a going concern.
We may seek to obtain additional capital
through the sale of debt or equity financings or other arrangements to fund operations; however, there can be no assurance that
we will be able to raise needed capital under acceptable terms, if at all. The sale of additional equity may dilute existing stockholders
and newly issued shares may contain senior rights and preferences compared to currently outstanding shares of common stock. Issued
debt securities may contain covenants and limit our ability to pay dividends or make other distributions to stockholders.
We
may not generate sufficient cash flows to cover our operating expenses.
As
noted above, we have incurred recurring losses since inception and expect to continue to incur losses as a result of costs and
expenses related to our research and continued development of our technology and our corporate general and administrative expenses.
Our limited capital resources and operations to date have been substantially funded through sales of our securities. As of September
30, 2020, we had negative working capital of approximately $6.9 million, cash of approximately $601,000, shareholders’ deficit
of approximately $6.6 million and an accumulated deficit of approximately $32.7 million. In the event that we are unable to generate
sufficient cash from our operating activities or raise additional funds, we may be required to delay, reduce or severely curtail
our operations or otherwise impede our on-going business efforts, which could have a material adverse effect on our business,
operating results, financial condition and long-term prospects.
We
have never declared a cash dividend and do not intend to declare a cash dividend in the foreseeable future.
We
have never declared or paid cash dividends on our common stock. Payment of dividends on our common stock is within the discretion
of our Board of Directors and will depend upon our future earnings, capital requirements, financial condition and other relevant
factors. We do not anticipate declaring or paying any cash dividends on our common stock in the foreseeable future.
We
do not directly manufacture products using Electrokinetic technology. We currently rely upon the activities of our licensees and
their customers in order to generate revenue.
We do not directly manufacture products
using electrokinetic (EK) technology. We currently depend upon the activities of our licensees in order be able to generate revenue.
It will be up to our licensees to decide when and if they will introduce products using electrokinetic technology, we cannot predict
when and if our licensees will generate substantial sales of such products. We have agreements with two companies to evaluate
our electrokinetic technology to determine the feasibility to manufacture and distribute the CEK Film to the automotive market.
Other companies are also evaluating electrokinetic technology for use in various products. While we expect that our licensees
would be primarily responsible for manufacturing and marketing electrokinetic products and components, we are also engaging in
market development activities to support partners to build the smart glass industry. We cannot control whether or not our licensees
will develop electrokinetic products. There is no guarantee when or if our licensees will successfully produce any commercial
product using electrokinetic technology in sufficient quantities to make the Company profitable.
Electrokinetic
products face intense competition, which could affect our ability to increase our revenues.
The
market for electrokinetic products is intensely competitive and we expect competition to increase in the future. We compete based
on the functionality and the quality of our product. Many of our current and potential competitors have significantly greater
financial, technical, marketing and other resources than we have. In addition, many of our competitors have well-established relationships
with our current and potential customers and have extensive knowledge of our industry. If our competitors develop new technologies
or new products, improve the functionality or quality of their current products, or reduce their prices, and if we are unable
to respond to such competitive developments quickly either because our research and development efforts do not keep pace with
our competitors or because of our lack of financial resources, we may be unable to compete effectively.
Declining
production of automobiles and real estate could harm our business.
Our
commercialization efforts could be negatively impacted if the global production of automobiles and real estate construction declines
significantly. If such commercialization is reduced, our revenues, results of operations and financial condition could be negatively
impacted.
We
are dependent on key personnel.
Our
continued success will depend, to a significant extent, on the services of our directors, executive management team, key personnel
and certain key scientists. If one or more of these individuals were to leave the Company, there is no guarantee that we could
replace them with qualified individuals in a timely or economically satisfactory manner or at all. The loss or unavailability of
any or all of these individuals could harm our ability to execute our business plan, maintain important business relationships
and complete certain product development initiatives, which would have a material adverse effect on our business, results of operations
and financial conditions.
Dependence
on electrokinetic technology.
Because electrokinetic technology is
the only technology we work with, our success depends upon the viability of electrokinetic technology which has yet to be fully
proven. We have not fully ascertained the performance and long-term reliability of our technology, and therefore there is no guarantee
that our technology will be successfully incorporated into all of the products which we are targeting for use of electrokinetic
technology. We expect that different product applications for electrokinetic technology will have different performance and reliability
specifications. We expect that our licensees will primarily be responsible for reliability testing, but that we may also continue
to do reliability testing so that we can more effectively focus our research and development efforts toward constantly improving
the performance characteristics and reliability of products using electrokinetic technology.
Our
patents and other protective measures may not adequately protect our proprietary intellectual property, and we may be infringing
on the rights of others.
Our
intellectual property, particularly our proprietary rights in our electrokinetic technology, is critical to our success. We have
licensed various patents, and filed other patent applications, for various applications and aspects of our electrokinetic technology.
In addition, we generally enter into confidentiality and invention agreements with our employees and consultants. Such patents
and agreements and various other measures we take to protect our intellectual property from use by others may not be effective
for various reasons generally applicable to patents and their granting and enforcement. In addition, the costs associated with
enforcing patents, confidentiality and invention agreements or other intellectual property rights may be expensive. Our inability
to protect our proprietary intellectual property rights or gain a competitive advantage from such rights could harm our ability
to generate revenues and, as a result, our business and operations.
The
extent to which the coronavirus (“COVID-19”) outbreak impacts our business, results of operations and financial condition
will depend on future developments, which cannot be predicted.
The
COVID-19 pandemic has caused us to modify our business practices (including employee travel, employee work locations, and cancellation
of physical participation in meetings, events and conferences), and we may take further actions as may be required by government
authorities or that we determine are in the best interests of our employees, customers and business partners. There is no certainty
that such measures will be sufficient to mitigate the risks posed by the virus or otherwise be satisfactory to government authorities.
The
extent to which COVID-19 impacts our business, results of operations and financial condition will depend on future developments,
which are uncertain and cannot be predicted, including, but not limited to, the duration and spread of the outbreak, its severity,
the actions to contain the virus or treat its impact, and how quickly and to what extent normal economic and operating conditions
can resume. Even after the coronavirus outbreak has subsided, we may continue to experience materially adverse impacts to our
business as a result of its global economic impact, including any recession that has occurred or may occur in the future.
Risks
Related to our Common Stock and this Offering
If
our shares of common stock become subject to the penny stock rules, it would become more difficult to trade our shares.
The
SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are
generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges
or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect
to transactions in such securities is provided by the exchange or system. If we do not obtain or retain a listing on the Exchange
or another national securities exchange and if the price of our common stock is less than $5.00, our common stock could be deemed
a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from
those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock
rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must
make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s
written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny
stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect
of reducing the trading activity in the secondary market for our common stock, and therefore stockholders may have difficulty
selling their shares.
There
has been no consistent active trading market for our common stock, and public trading of our common stock may continue to be inactive
and fluctuate substantially.
There
has never been a consistent active trading market for our common stock. Our common stock currently trades over-the-counter on
the QB tier of OTC Markets Group, Inc. under the symbol “CRKN.” We have applied to list our common stock on the Exchange
under the symbol “CRKN.” There is no assurance that the trading market for our common stock will become more active
or liquid. Furthermore, there can be no assurance any broker will be interested in trading our stock. Therefore, it may be difficult
to sell your shares of common stock if you desire or need to sell them.
Moreover,
the trading price of our common stock has fluctuated substantially over the past few years, and there remains a significant risk
that our common stock price may continue to fluctuate substantially in the future in response to various factors, including any
material variations in our periodic operating results, departures or additions of management or other key personnel, announcements
of acquisitions, mergers, or new technology or patents, new product developments, significant litigation matters, gain or loss
of significant customers, significant capital transactions, substantial sales of our common stock in our trading market, and general
and specific market and economic conditions.
We
anticipate effecting a reverse stock split of our outstanding common stock prior to the closing of this offering.
We
expect that the reverse stock split will increase the market price of our common stock while our stock is trading and enable us
to meet the minimum market price requirement of the listing rules of the Exchange. However, the effect of a reverse stock split
upon the market price of our common stock cannot be predicted with certainty, and the results of reverse stock splits by companies
in similar circumstances have been varied. It is possible that the market price of our common stock following the reverse stock
split will not increase sufficiently for us to be in compliance with the minimum market price requirement of the Exchange, or
if it does, that such price will be sustained. If we are unable to meet the minimum market price requirement, we may be unable
to list our shares on the Exchange, in which case such an offering may not be completed.
Even
if the reverse stock split achieves the requisite increase in the market price of our common stock, there can be no assurance
that we will be approved for listing on the Exchange or able to comply with other continued listing standards of the Exchange.
Even
if the market price of our common stock increases sufficiently so that we comply with the minimum market price requirement, we
cannot assure you that we will be able to comply with the other standards that we are required to meet in order to be approved
for listing on the Exchange or maintain a listing of our common stock on the Exchange. Our failure to meet these requirements
may result in our common stock being delisted from the Exchange.
The
reverse stock split could decrease the liquidity of our stock.
The
reverse stock split could adversely affect the liquidity of our common stock given the reduced number of shares that will be outstanding
after the reverse stock split. In addition, the reverse stock split could increase the number of stockholders who own odd lots
(less than 100 shares) of our common stock, creating the potential for such stockholders to experience an increase in the cost
of selling their shares and greater difficulty effecting such sales.
Even
after the reverse stock split, the trading price of our common stock may not be high enough to attract new investors, including
institutional investors, and may not satisfy the investing requirements of those investors. Consequently, the trading liquidity
of our common stock may not improve.
Even
after the reverse stock split, there can be no assurance that the reverse stock split would result in a share price that will
attract new investors, including institutional investors, or that the share price will satisfy the investing requirements of those
investors. As a result, the trading liquidity of our common stock may not necessarily improve, our share price may decline and
you may lose all or part of your investment.
Our
stock price may be volatile, which could result in substantial losses to investors and litigation.
In
addition to changes to market prices based on our results of operations and the factors discussed elsewhere in this “Risk
Factors” section, the market price of and trading volume for our common stock may change for a variety of other reasons,
not necessarily related to our actual operating performance. The capital markets have experienced extreme volatility
that has often been unrelated to the operating performance of particular companies. These broad market fluctuations
may adversely affect the trading price of our common stock. In addition, the average daily trading volume of the securities
of small companies can be very low, which may contribute to future volatility. Factors that could cause the market
price of our common stock to fluctuate significantly include:
| |
● |
the
results of operating and financial performance and prospects of other companies in our industry; |
| |
|
|
| |
● |
strategic
actions by us or our competitors, such as acquisitions or restructurings; |
| |
|
|
| |
● |
announcements
of innovations, increased service capabilities, new or terminated customers or new, amended or terminated contracts by our
competitors; |
| |
|
|
| |
● |
the
public’s reaction to our press releases, other public announcements, and filings with the Securities and Exchange Commission; |
| |
|
|
| |
● |
lack
of securities analyst coverage or speculation in the press or investment community about us or market opportunities in the
smart glass industry; |
| |
● |
changes
in government policies in the United States and, as our international business increases, in other foreign countries; |
| |
● |
changes
in earnings estimates or recommendations by securities or research analysts who track our common stock or failure of our actual
results of operations to meet those expectations; |
| |
● |
market
and industry perception of our success, or lack thereof, in pursuing our growth strategy; |
| |
● |
changes
in accounting standards, policies, guidance, interpretations or principles; |
| |
● |
any
lawsuit involving us, our services or our products; |
| |
● |
arrival
and departure of key personnel; |
| |
● |
sales
of common stock by us, our investors or members of our management team; and |
| |
● |
changes
in general market, economic and political conditions in the United States and global economies or financial markets, including
those resulting from natural or man-made disasters. |
Any
of these factors, as well as broader market and industry factors, may result in large and sudden changes in the trading volume
of our common stock and could seriously harm the market price of our common stock, regardless of our operating performance. This
may prevent you from being able to sell your shares at or above the price you paid for your shares, if at all. In addition, following
periods of volatility in the market price of a company’s shares, stockholders often institute securities class action litigation
against that company. Our involvement in any class action suit or other legal proceeding could divert our senior management’s
attention and could adversely affect our business, financial condition, results of operations and prospects.
The
sale or availability for sale of substantial amounts of our common stock could adversely affect the market price of our common
stock.
Sales
of substantial amounts of shares of our common stock after the completion of the offering, or the perception that these sales
could occur, could adversely affect the market price of our common stock and could impair our future ability to raise capital
through common stock offerings. Our executive officers and directors beneficially own, collectively, a substantial percentage
of our outstanding common stock. If one or more of them were to sell a substantial portion of the shares they hold, it could cause
our stock price to decline.
In addition, as of September 30, 2020, there were outstanding
warrants to purchase an aggregate of 2,520,051 shares of our common stock at a weighted-average exercise price of $2.23 per share,
all of which were exercisable as of such date. As of September 30, 2020, we also had outstanding options to purchase 5,561,367
shares of our common stock, all of which have vested, with strike prices ranging from $0.15 per share to $3.00 per share. The exercise
of options or warrants at prices below the market price of our common stock could adversely affect the price of shares of our common
stock. Additional dilution may result from the issuance of shares of our capital stock in connection with acquisitions or in connection
with other financing efforts. Any issuance of our common stock that is not made solely to then-existing stockholders proportionate
to their interests, such as in the case of a stock dividend or stock split, will result in dilution to each stockholder.
We
are controlled by a small group of our existing stockholders, whose interests may differ from other stockholders. Our executive
officers and directors will significantly influence our activities, and their interests may differ from your interests as a stockholder.
Our
executive officers and directors beneficially own, collectively, a substantial percentage of our outstanding common stock.
Accordingly,
these stockholders have had, and will continue to have, significant influence in determining the outcome of any corporate transaction
or any other matter submitted for approval to our stockholders, including mergers, consolidations and the sale of our assets,
director elections and other significant corporate actions. They will also have significant influence in preventing
or causing a change in control of our company. In addition, without the consent of these stockholders, we could be
prevented from entering into transactions that could be beneficial to us. The interests of these stockholders may differ
from your interests as a stockholder, and they may act in a manner that advances their best interests and not necessarily those
of other stockholders.
Our
certificate of incorporation and bylaws, and certain provisions of Delaware corporate law, contain provisions that could delay
or prevent a change in control even if the change in control would be beneficial to our stockholders.
Delaware
law, as well as our certificate of incorporation and bylaws, contain anti-takeover provisions that could delay or prevent a change
in control of our company, even if the change in control would be beneficial to our stockholders. These provisions could lower
the price that future investors might be willing to pay for shares of our common stock. These anti-takeover provisions:
| |
● |
authorize
our board of directors to create and issue, without stockholder approval, preferred stock, thereby increasing the number of
outstanding shares, which can deter or prevent a takeover attempt; |
| |
● |
prohibit
stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders; |
| |
● |
establish
a three-tiered classified board of directors requiring that not all members of our board be elected at one time; |
| |
● |
establish
a supermajority requirement to amend our amended and restated bylaws and specified provisions of our amended and restated
certificate of incorporation; |
| |
● |
prohibit
cumulative voting in the election of directors, which would otherwise allow less than a majority of stockholders to elect
director candidates; |
| |
● |
establish
limitations on the removal of directors; |
| |
● |
empower
our board of directors to fill any vacancy on our board of directors, whether such vacancy occurs as a result of an increase
in the number of directors or otherwise; |
| |
● |
provide
that our board of directors is expressly authorized to adopt, amend or repeal our bylaws; |
| |
● |
provide
that our directors will be elected by a plurality of the votes cast in the election of directors; |
| |
● |
establish
advance notice requirements for nominations for election to our board of directors or for proposing matters that can be acted
on by our stockholders at stockholder meetings; and |
| |
● |
limit
the ability of our stockholders to call special meetings of stockholders. |
If
equity research analysts do not publish research or reports about our business, or if they issue unfavorable commentary or downgrade
our common stock, the market price of our common stock will likely decline.
The
trading market for our common stock will rely in part on the research and reports that equity research analysts, over whom we
have no control, publish about us and our business. We may never obtain research coverage by securities and industry
analysts. If no securities or industry analysts commence coverage of our company, the market price for our common stock could
decline. In the event we obtain securities or industry analyst coverage, the market price of our common stock could decline if
one or more equity analysts downgrade our common stock or if those analysts issue unfavorable commentary, even if it is inaccurate,
or cease publishing reports about us or our business.
Our
management has broad discretion as to the use of the net proceeds from this offering.
Our
management will have broad discretion in the application of the net proceeds. Accordingly, you will have to rely upon the judgment
of our management with respect to the use of these proceeds. Our management may spend a portion or all of the net proceeds from
this offering in ways that holders of our common stock may not desire or that may not yield a significant return or any return
at all. Our management not applying these funds effectively could harm our business. Pending their use, we may also invest the
net proceeds from this offering in a manner that does not produce income or that loses value. Please see “Use of Proceeds”
below for more information.
You
will experience immediate and substantial dilution as a result of this offering.
As of September 30, 2020, our net tangible
book value (deficit) was approximately $(7.3 million), or $(0.77) per share. Since the effective price per share of our common
stock being offered in this offering is substantially higher than the net tangible book value per share of our common stock, you
will suffer substantial dilution with respect to the net tangible book value of the common stock you purchase in this offering.
Based on the assumed public offering price of $16.50 per share of common stock being sold in this offering, and our net tangible
book value per share as of September 30, 2020, if you purchase shares of common stock in this offering, you will suffer immediate
and substantial dilution of $15.61 per share with respect to the net tangible book value of the common stock (assuming no exercise
of the underwriters’ option to purchase additional shares). See the section titled “Dilution” for a more
detailed discussion of the dilution you will incur if you purchase shares of common stock in this offering.
Assuming
we obtain an Exchange listing, we will incur material increased costs and become subject to additional regulations and requirements.
As
a newly Exchange-listed public company, we will incur material additional legal, accounting and other expenses including recruiting
and retaining qualified independent directors, payment of annual Exchange fees, and satisfying Exchange standards for companies
listed with it. If our common stock is listed on an Exchange, we must meet certain financial and liquidity criteria to maintain
such listing. If we violate Exchange listing requirements, our common stock may be delisted. If we fail to meet any of the Exchange’s
listing standards, our common stock may be delisted. In addition, our board of directors may determine that the cost of maintaining
our listing on a national securities exchange outweighs the benefits of such listing. A delisting of our common stock from the
Exchange may materially impair our stockholders’ ability to buy and sell our common stock and could have an adverse effect
on the market price of, and the efficiency of the trading market for, our common stock. The delisting of our common stock could
significantly impair our ability to raise capital and the value of your investment.
We
do not anticipate paying any dividends on our common stock for the foreseeable future.
We
have not paid any dividends on our common stock to date, and we do not anticipate paying any such dividends in the foreseeable
future. We anticipate that any earnings experienced by us will be retained to finance the implementation of our operational business
plan and expected future growth.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some
of the statements under “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and
Analysis of Financial Condition and Results of Operations,” “Business,” and elsewhere in this prospectus constitute
forward-looking statements. These statements involve risks known to us, significant uncertainties, and other factors which may
cause our actual results, levels of activity, performance, or achievements to be materially different from any future results,
levels of activity, performance, or achievements expressed or implied by those forward-looking statements.
You
can identify forward-looking statements by the use of the words “may,” “will,” “should,” “could,”
“expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,”
“intends,” “potential,” “proposed,” or “continue” or the negative of those terms.
These statements are only predictions. In evaluating these statements, you should specifically consider various factors, including
the risks outlined above. These factors may cause our actual results to differ materially from any forward-looking statement.
Although
we believe that the exceptions reflected in the forward-looking statements are reasonable, we cannot guarantee future results,
levels of activity, performance or achievements.
USE
OF PROCEEDS
After deducting the estimated underwriting
discounts and commissions and offering expenses payable by us, we expect to receive net proceeds of approximately $15,370,000
from this offering (or approximately $17,716,000 if the underwriters’ option to purchase additional shares is exercised
in full), based on an assumed public offering price of $16.50 per share (the last reported sale price of our common stock on the
OTCQB on January 21, 2021, as adjusted for the one-for-three (1:3) reverse stock split of our common stock that will become effective
on the date our common stock is listed on the Exchange). We intend to use these net proceeds for capital expenditures, working
capital and the purchase of seven patents that we currently license from HP for an aggregate purchase price of $1,475,000) and
other general corporate purposes.
Each $1.00 increase or decrease in
the assumed public offering price of $16.50 per share would increase (decrease) the net proceeds that we receive from this offering
by approximately $947,879, assuming that the number of shares of common stock offered by us, as set forth on the cover page of
this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering
expenses payable by us. Similarly, each increase (decrease) of 100,000 shares of common stock offered by us in this offering would
increase (decrease) the net proceeds that we receive from this offering by approximately $1,518,000, assuming the assumed public
offering price remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering
expenses payable by us.
The
precise amounts that we will devote to each of the foregoing items, and the timing of expenditures, will vary depending on numerous
factors.
The
following table sets forth a breakdown of our estimated use of our net proceeds as we currently expect to use them.
| | |
Amount | |
| Capital expenditures | |
$ | 1,000,000 | |
| Purchase of patents | |
$ | 1,475,000 | |
| Working
capital | |
$ | 12,895,000 | |
| Total use
of proceeds | |
$ | 15,370,000 | |
In the event that the underwriters
exercise the over-allotment option, we intend to use such additional net proceeds (up to $17,716,000) for working capital and
other general corporate purposes.
The
expected use of net proceeds from this offering represents our intentions based upon our current plans and business conditions,
which could change in the future as our plans and business conditions evolve and change. The amounts and timing of our actual
expenditures, specifically with respect to working capital, may vary significantly depending on numerous factors. As a result,
our management will retain broad discretion over the allocation of the net proceeds from this offering.
MARKET
FOR COMMON EQUITY AND RELATED STOCKHOLDERS MATTERS
Market
for Common Stock
Our
common stock began trading on the OTCQB under the symbol “CRKN” on June 9, 2020. Prior to that time, there was no
public market for our common stock.
Holders
We are authorized to issue up to 200,000,000
shares of common stock, par value $0.0001 per share, and 50,000,000 shares of preferred stock. As of January 11, 2021, there were
7,649,346 shares of common stock issued and outstanding and 107 shareholders of record. The number of record holders does not
include persons who held shares of our common stock in “street name” accounts through brokers, banks and other financial
institutions.
Warrants
and Stock Options
As of January 11, 2021, we have options
and warrants outstanding for the purchase of 13,952,483 shares of our common stock.
Dividend
Policy
We
have never paid or declared any dividend on our common stock and we do not anticipate paying cash dividends in the foreseeable
future.
Securities
authorized for issuance under equity compensation plans
As of September 30, 2020, there are
7,333,333 shares authorized under the Equity Incentive plan.
Options
granted in the future under the Equity Incentive Plan are within the discretion of our board of directors. The following table
summarizes the number of shares of our common stock authorized for issuance under our equity compensation plans.
| Plan Category | |
(a)
Number of
Securities
to be Issued
Upon
Exercise of
Outstanding Options | | |
(b)
Weighted-
Average
Exercise
Price of
Outstanding
Options | | |
(c)
Number of
Securities
Remaining
Available for
Future
Issuance
Under Equity
Compensation
Plans
(excluding
securities
reflected in
column
(a)) | |
| Equity compensation plans approved by security holders | |
| 5,561,367 | | |
| N/A | | |
| 7,333,333 | |
| Equity compensation plans not approved by security holders | |
| 0 | | |
| N/A | | |
| 0 | |
| Total | |
| 5,561,367 | | |
| N/A | | |
| 7,333,333 | |
CAPITALIZATION
The
following table sets forth our capitalization as of September 30, 2020:
| |
● |
on
an actual basis; and |
| |
● |
on
a pro forma basis to reflect (a) the sale of 1,030,303 shares of common stock by us in this offering at an assumed price to
the public of $16.50 per share (the last reported sale price of our common stock on the OTCQB on January 21, 2021, as adjusted
for the one-for-three (1:3) reverse stock split of our common stock that will become effective on the date our common stock
is listed on the Exchange), resulting in net proceeds to us of $15,370,000 after deducting (i) underwriting discounts and
commissions of $1,360,001.28 and (ii) our estimated other offering expenses of $270,000, (b) the conversion by a convertible
noteholder of an aggregate of $343,423.02 of debt pursuant to such notes into 360,111 shares common stock and 251 shares of
Series A Preferred Stock simultaneously with the closing of this offering and (c) the conversion by a convertible noteholder
of an aggregate of $1,496,751.82 of debt pursuant to such notes into 173,111 shares of common stock and 1,443.41 shares of
Series B Preferred Stock simultaneously with the closing of this offering. |
The
pro forma information below is illustrative only and our capitalization following the completion of this offering is subject to
adjustment based on the public offering price of the shares of common stock and other terms of this offering determined at pricing.
You should read this table together with our consolidated financial statements and the related notes included elsewhere in this
prospectus and the information under “Management’s Discussion and Analysis of Financial Condition and Results of
Operations”.
Offering (1,030,303
Shares of Common Stock)
| | |
Actual(1) | | |
Pro
Forma
Offering
Amount | |
| | |
(Unaudited) | | |
(Unaudited)(2) | |
| Cash and
Cash Equivalents | |
$ | 600,924 | | |
$ | 15,970,924 | |
| Notes payable, net of
debt discount of $501,370 | |
$ | 2,176,900 | | |
| 711,561 | |
| Stockholders (deficit) equity: | |
| | | |
| | |
| Preferred
Stock, $0.0001 par value per share, 50,000,000 shares authorized(1)(2) | |
| 0 | | |
| 0 | |
| Common
Stock, $0.0001 par value per share, 200,000,000 shares authorized(1)(2) | |
$ | 2,864 | | |
$ | 1,109 | |
| Additional Paid-in Capital | |
$ | 26,082,835 | | |
$ | 43,283,845 | |
| Treasury Stock | |
$ | 0 | | |
$ | 0 | |
| Accumulated deficit | |
$ | (32,676,871 | ) | |
$ | (32,676,871 | ) |
| Total Stockholders’
Equity (Deficit) | |
$ | (6,591,172 | ) | |
$ | 10,608,083 | |
| Total Capitalization | |
$ | (4,414,272 | ) | |
$ | 11,319,644 | |
| (1) |
9,546,621
shares of common stock and 0 shares of preferred stock issued and outstanding as of September 30, 2020. |
| (2) |
11,089,329
shares of common stock and 1,675 shares of preferred stock issued and outstanding (pro forma) as of September 30, 2020 after
the completion of this offering. |
Each $1.00 increase (decrease) in the
assumed offering price per security of $16.50, assuming no change in the number of shares of common stock to be sold, would increase
(decrease) the net proceeds that we receive in this offering and each of total stockholders’ equity and total capitalization
by approximately $947,879, after deducting (i) estimated underwriting discounts and commissions and (ii) offering expenses, in
each case, payable by us. Similarly, an increase (decrease) of 100,000 shares of common stock offered by us in this offering,
assuming no change in the offering price, would increase (decrease) the net proceeds that we receive in this offering and each
of total stockholders’ equity and total capitalization by approximately $1,518,000, after deducting (i) estimated underwriting
discounts and commissions and (ii) offering expenses, in each case, payable by us.
The
table above excludes the following shares:
| |
●
|
|
7,333,333
shares of common stock reserved for issuance under our 2016 Equity Incentive Plan; |
| |
●
|
5,561,367 shares
of common stock issuable upon the exercise of options (of which 4,750,055 have vested) at a weighted average exercise price
of $1.75 as of September 30, 2020; |
| |
● |
2,520,051 shares
of common stock issuable upon the exercise of warrants; and |
| |
● |
410,453 shares
of common stock issuable upon the conversion of outstanding convertible promissory notes (not including shares of preferred
stock to be issued to certain convertible noteholders). |
DILUTION
Dilution
in net tangible book value per share to new investors is the amount by which the offering price paid by the purchasers of the
shares of common stock sold in this offering exceeds the pro forma net tangible book value per share of common stock after this
offering. Net tangible book value per share is determined at any date by subtracting our total liabilities from the total book
value of our tangible assets and dividing the difference by the number of shares of common stock deemed to be outstanding at that
date.
The net tangible book value of our
common stock as of September 30, 2020 was approximately $(7.3 million), or approximately $(0.77) per share.
Pro forma as adjusted net tangible
book value (deficit) dilution per share to new investors represents the difference between the amount per security paid by purchasers
of the shares of common stock in this offering and the pro forma as adjusted net tangible book value per share of our common stock
immediately after completion of this offering. Investors participating in this offering will incur immediate, substantial dilution.
After giving effect to our sale of 1,030,303 shares of common stock in this offering at an assumed public offering price of $16.50
per share (the last reported sale price of our common stock on the OTCQB on January 21, 2021, as adjusted for the one-for-three
(1:3) reverse stock split of our common stock that will become effective on the date our common stock is listed on the Exchange),
and after deducting the estimated underwriting discounts and commissions and estimated offering expenses, our pro forma as adjusted
net tangible book value as of September 30, 2020 would have been approximately $9,891,907 or approximately $0.89 per share. This
amount represents an immediate increase in pro forma net tangible book value of $0.89 per share to existing stockholders and an
immediate dilution in pro forma net tangible book value of $15.61 per share to purchasers of shares of common stock in this offering,
as illustrated in the following table.
The
following table illustrates the estimated dilution on a per share basis:
| Assumed public offering price per
share | |
$ | 16.50 | |
| Net tangible book value (deficit) per share as
of September 30, 2020 | |
$ | (0.77 | ) |
| Increase in net tangible book value per share
after this offering | |
$ | 1.66 | |
| Pro forma net tangible book value per share after
this offering | |
$ | 0.89 | |
| Dilution in net tangible book value per share
to new investors | |
$ | 15.61 | |
The following table sets forth, assuming
the sale of 1,030,303 shares of common stock in this offering, as of September 30, 2020, the total number of shares of common
stock previously issued and sold to existing investors, the total consideration paid for the foregoing and the average price per
share of common stock, before deducting estimated underwriting discounts and commissions and offering expenses, in each case payable
by us. As the table shows, new investors purchasing shares of common stock in this offering may in certain circumstances pay an
average price per share substantially higher than the average price per share of common stock paid by our existing stockholders.
| September
30, 2020 | |
Number
of
Shares | | |
Purchased
Percentage | | |
Total
Consideration
Amount | | |
Total
Consideration
Percent | | |
Average
Price Per
Share | |
| | |
| | |
| | |
| | |
| | |
| |
| Existing Stockholders | |
| 9,546,621 | | |
| 86.09 | % | |
$ | 7,200,345 | | |
| 27.66 | % | |
$ | 1.26 | |
| Conversion of Notes | |
| 512,405 | | |
| 4.62 | % | |
$ | 1,829,255 | | |
| 7.03 | % | |
$ | 3.57 | |
| New Investors | |
| 1,030,303 | | |
| 9.29 | % | |
$ | 17,000,016 | | |
| 65.31 | % | |
$ | 16.50 | |
| Total: | |
| 11,089,329 | | |
| 100.0 | % | |
$ | 26,029,600 | | |
| 100.0 | % | |
$ | 2.35 | |
A $1.00 increase (decrease) in the
assumed public offering price of $16.50 per share assuming the number of shares of common stock offered by us remains the same
and after deducting estimated underwriting discounts and commissions and offering expenses payable by us would increase (decrease)
our pro forma net tangible book value (deficit), as adjusted to give effect to this offering, to $0.09 per share.
Similarly, a (decrease) of 100,000
shares of common stock offered by us assuming the public offering price of $16.50 per share remains the same and after deducting
estimated underwriting discounts and commissions and offering expenses payable by us would increase our pro forma net tangible
book value (deficit), as adjusted to give effect to this offering, to $(0.13) per share. In addition, a 100,000 share increase
would decrease the dilution to new investors by $0.13 per share.
The information discussed above, which
takes into account a 1-for-3 reverse stock split of our common stock, is illustrative only and will adjust based on the actual
public offering price, the actual number of shares of common stock that we offer in this offering, and the other terms of this
offering determined at pricing.
The table above excludes the following
shares:
| |
●
|
7,333,333
shares of common stock reserved for issuance under our 2016 Equity Incentive Plan; |
| |
●
|
5,561,367 shares
of common stock issuable upon the exercise of options (of which 4,750,055 have vested) at a weighted average exercise price
of $1.75 as of September 30, 2020; |
| |
● |
2,520,051 shares
of common stock issuable upon the exercise of warrants; and |
| |
● |
410,453 shares
of common stock issuable upon the conversion of outstanding convertible promissory notes (not including shares of preferred
stock to be issued to certain convertible noteholders). |
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The
following discussion and analysis of our financial condition and results of operations should be read in conjunction with the
financial statements and related notes thereto included elsewhere in this report. This discussion contains forward-looking statements
that involve risks and uncertainties. Our actual results could differ materially from those discussed below. Factors that could
cause or contribute to such differences include, but are not limited to, those identified below and those discussed in the section
titled “Risk Factors” included elsewhere in this report.
Management’s
plans and basis of presentation:
The
Company was incorporated in the State of Delaware on April 20, 2015. Effective January 14, 2016, the Company’s name was
changed to 3D Nanocolor Corp. (“3D Nanocolor”) from 2D Nanocolor Corp. Subsequently, effective October 6, 2017, the
Company’s name was changed to Crown Electrokinetics Corp. from 3D Nanocolor Corp.
The
Company is commercializing technology for smart or dynamic glass. The Company’s electrokinetic glass technology is an advancement
on microfluidic technology that was originally developed by Hewlett-Packard Company.
Crown’s
Research & Development Operation currently occupies 1,700 square feet of space, located on the HP Inc. campus in Corvallis,
Oregon in the Advanced Technology and Manufacturing Institute (ATAMI). ATAMI is an academic-industrial research center and business
incubator designed to provide an advanced materials development environment to private sector partner tenants performing research
and development. The facility includes access to shared state-of-the-art tooling capabilities. ATAMI has grown to 80,000 square
feet since its inception in 2004 and now offers Crown all the space requirements it needs for the foreseeable future.
On
November 15, 2017, the Company entered into a license agreement with Glass Manufacturer. The Glass Manufacturer agreement provides
that the Company will provide samples to be used by Glass Manufacturer for the sole purpose of determining the feasibility of
integrating the Company’s film technology in Glass Manufacturer’s auto and train glass products. The Company began
performing development activities in April of 2018. On February 1, 2019, the Company and Glass Manufacturer entered into a new
license agreement, terminating the prior agreement, which was further extended on November 14, 2019. Under such new license agreement,
the Company will provide samples to be used by Glass Manufacturer to evaluate the appearance of and measure optical properties
of the Company’s film technology. At Glass Manufacturer’s option, the Company will provide additional samples to be
used by Glass Manufacturer to measure the durability of such sample for the purpose of determining the feasibility of integrating
the Company’s film technology in Glass Manufacturer’s auto and train glass products. The performance related to the
new agreement is a continuation of the work being performed as of April 2018. On November 14, 2019, the Company entered into a
new agreement with Glass Manufacturer, which terminates the February 1, 2019 agreement as of June 16, 2019, (the “Effective
Date”) of the new agreement. Under the terms of the new agreement, Glass Manufacturer will pay the Company $0.1 million
within 60 days of the Effective Date. On December 10, 2019, the Company received the $0.1 million payment from Glass Manufacturer
and the Company delivered three pieces of updated samples to Glass Manufacturer on September 28, 2020.
On
August 23, 2017, the Company entered into a collaborative agreement with Film Manufacturer. The Film Manufacturer agreement provides
that the Company and Film Manufacturer will jointly develop electrokinetic films and determine their suitability for commercial
use in applied films and interlayers for automobile windows. The Company and Film Manufacturer will be exchanging IP for the development
of the films. The Company began performing development activities in April of 2018.
Results
of Operations for the three months ended September 30, 2020 and 2019 (income):
| | |
Three Months Ended
September 30, | |
| | |
2020 | | |
2019 | |
| Revenue | |
$ | - | | |
$ | - | |
| Cost of revenue | |
| - | | |
| 153,500 | |
| Research and development | |
| 423,174 | | |
| 750,395 | |
| Selling, general and administrative | |
| 1,659,612 | | |
| 1,492,483 | |
| Other expense | |
| 3,683,696 | | |
| 404,802 | |
| Net Loss | |
$ | 5,766,482 | | |
$ | 2,801,180 | |
Revenue
The
Company did not recognize revenue for the three months ended September 30, 2020 and 2019. We are not able to estimate the total
amount of development service under an efforts-based perspective and, therefore, the amount of performance that will be required
in our contracts cannot be reliably estimated under the proportional performance revenue recognition model. Accordingly, we recognize
revenue up to the amount of costs incurred.
Cost
of Revenue
There
was no cost of revenue recognized during the three months ended September 30, 2020. The cost of revenue for the three months ended
September 30, 2019, was approximately $154,000 and consists of approximately $125,000 related to the costs incurred with
respect to our contract with Film Manufacturer and approximately $29,000 with respect to our contract with Glass Manufacturer.
Research
and Development
Research
and development expenses were $0.4 million for the three months ended September 30, 2020 compared to $0.8 million for the three
months ended September 30, 2019. The decrease of $0.4 million is primarily related to lower stock-based compensation expenses
recognized for stock options granted to our employees and officers.
Selling,
General and Administrative
Selling,
general and administrative (“SG&A”) expenses were $1.7 million and $1.5 million for the three months ended September
30, 2020 and 2019, respectively. The $0.2 million increase in SG&A expenses is primarily due to $0.6 million of stock-based
compensation expense related to stock options granted during the three months ended September 30, 2020 to an officer of the Company
and to an advisor, $0.1 million of increased payroll and related expenses for new hires, offset by lower stock-based compensation
expense related to our restricted stock of approximately $0.6 million.
Other
Expense
Other
expense was approximately $3.7 million and $0.4 million for the three months ended September 30, 2020 and 2019, respectively.
The $3.3 million increase is primarily due to increased interest expense of $0.6 million related to our convertible notes, loss
on exchange of our convertible notes for common stock and warrants of $1.5 million, change in fair value of our warrant liability
of $0.9 million, loss on extinguishment of debt of $0.2 million, and $0.1 million of other expenses.
Results
of Operations for the six months ended September 30, 2020 and 2019 (income):
| | |
Six Months Ended
September 30, | |
| | |
2020 | | |
2019 | |
| Revenue | |
$ | - | | |
$ | - | |
| Cost of revenue | |
| - | | |
| (307,000 | ) |
| Research and development | |
| (1,795,696 | ) | |
| (1,070,766 | ) |
| Selling, general and administrative | |
| (9,597,169 | ) | |
| (2,868,391 | ) |
| Other expense | |
| (4,835,647 | ) | |
| (780,273 | ) |
| Net Loss | |
$ | (16,228,512 | ) | |
$ | (5,026,430 | ) |
Revenue
The
Company did not recognize revenue for the six months ended September 30, 2020 and 2019. We are not able to estimate the total
amount of development service under an efforts-based perspective and, therefore, the amount of performance that will be required
in our contracts cannot be reliably estimated under the proportional performance revenue recognition model. Accordingly, we recognize
revenue up to the amount of costs incurred.
Cost
of Revenue
There
was no cost of revenue recognized during the six months ended September 30, 2020. The cost of revenue for the six months ended
September 30, 2019, was $307,000 and consists of approximately $249,000 related to the costs incurred with respect to our contract
with Eastman and approximately $58,000 with respect to our contract with Asahi.
Research
and Development
Research
and development expenses were $1.8 million for the six months ended September 30, 2020 compared to $1.1 million for the six months
ended September 30, 2019. The increase of $0.7 million is primarily related to stock-based compensation expenses recognized for
stock options granted to our employees and officers.
Selling,
General and Administrative
SG&A
expenses were $9.6 million and $2.9 million for the six months ended September 30, 2020 and 2019, respectively. The $7.0 million
increase in SG&A expenses is primarily attributable to stock-based compensation of $3.6 million recognized with the issuance
of 800,000 shares of restricted stock to our chief executive officer, $3.1 million of stock-based compensation related to stock
options granted to our employees and officers and $0.1 million of increased payroll and related expenses for new hires.
Other
Expense
Other
expense was approximately $4.8 million and $0.8 million for the six months ended September 30, 2020 and 2019, respectively. The
$4.0 million increase is primarily due to increased interest expense of $1.4 million related to our convertible notes, loss on
exchange of our convertible notes for common stock and warrants of $1.5 million, change in fair value of our warrant liability
of $0.7 million, loss on extinguishment of debt of $0.2 million, and $0.1 million of other expenses.
Liquidity
Going
Concern
We
have incurred substantial operating losses since our inception, and we expect to continue to incur significant operating losses
for the foreseeable future, and may never become profitable. We had an accumulated deficit of approximately $32.7 million at September
30, 2020, a net loss of approximately $16.2 million, and approximately $2.8 million of net cash used in operating activities for
the six months ended September 30, 2020. We anticipate incurring additional losses until such time, if ever, that we can obtain
marketing approval to sell, and then generate significant sales, of our technology that is currently in development.
We
may seek to obtain additional capital through the sale of debt or equity financings or other arrangements to fund operations;
however, there can be no assurance that we will be able to raise needed capital under acceptable terms, if at all. The sale of
additional equity may dilute existing stockholders and newly issued shares may contain senior rights and preferences compared
to currently outstanding shares of common stock. Issued debt securities may contain covenants and limit our ability to pay dividends
or make other distributions to stockholders. If we are unable to obtain such additional financing, future operations would need
to be scaled back or discontinued. Due to the uncertainty in the Company’s ability to raise capital, we believe that there
is substantial doubt in our ability to continue as a going concern for twelve months from the date of issuance of the financial
statements.
Cash
Flows
| | |
Six Months Ended
September 30, | |
| | |
2020 | | |
2019 | |
| Cash and cash equivalents at the beginning of the period | |
$ | 48,307 | | |
$ | 99,447 | |
| Net cash used in operating activities | |
| (2,794,232 | ) | |
| (732,051 | ) |
| Net cash used in investing activities | |
| (29,051 | ) | |
| (26,603 | ) |
| Net cash provided by financing activities | |
| 3,375,900 | | |
| 659,207 | |
| Cash and cash equivalents at the end of the period | |
$ | 600,924 | | |
$ | - | |
Operating
Activities
For
the six months ended September 30, 2020, net cash used in operating activities was $2.8 million, which primarily consisted of
our net loss of $16.2 million, adjusted for non-cash expenses of $13.9 million including, $9.3 million of stock-based compensation
expenses, $2.0 million of amortization related to the debt discount recognized for our convertible notes payable, $1.5 million
recognized for the loss on exchange of our convertible notes for common stock and warrants, $0.7 million for the change in fair
value of our warrant liability, $0.2 million for the loss on extinguishment of debt, and $0.1 million of other non-cash expenses.
The net change in operating assets and liabilities was $0.4 million and was primarily due to decreases in accounts payable and
accrued expenses totaling $0.6 million, offset by a $0.3 million increase in accrued interest related to our convertible notes
and a $0.1 million increase to prepaid expenses and other current assets.
For
the six months ended September 30, 2019, net cash used in operating activities was $0.7 million, which primarily consisted of
our net loss of $5.0 million, adjusted for non-cash expenses of $3.5 million including, $2.9 million of stock-based compensation
expenses, $0.7 million of amortization related to the debt discount recognized for our convertible notes payable, off-set by $0.1
million for the change in fair value of our warrant liability. The net change in operating assets and liabilities was $0.8 million
and was primarily due to the increases in in accounts payable and accrued expenses totaling $0.6 million and increased accrued
interest of $0.2 million related to our convertible notes.
Investing
Activities
For
the six months ended September 30, 2020, net cash used in investing activities was approximately $29,000, related to the purchase
of computer equipment and computer software.
For
the six months ended September 30, 2019, net cash used in investing activities was approximately $27,000, related to the purchase
of computer equipment and computer software.
Financing
Activities
For
the six months ended September 30, 2020, net cash provided by financing activities was $3.4 million. The net cash provided is
primarily related to $2.1 million of proceeds received from the issuance of our senior secured convertible notes and the related
stock warrants, $1.6 million related to the net proceeds received from the issuance of the Company’s common stock and warrants,
and $0.2 million of proceeds received from our PPP loan, offset by $0.2 million for the repurchase of shares of our common stock
and $0.2 million for the repayment of our senior secured promissory note.
For
the six months ended September 30, 2019, net cash provided by financing activities was $0.7 million. The net cash provided is
primarily related to $0.6 million of proceeds received from the issuance of our senior secured convertible notes and the related
stock warrants.
Results
of Operations for the years ended March 31, 2020 and 2019
| | |
Years Ended March 31, | |
| | |
2020 | | |
2019 | |
| Revenue | |
$ | 100,000 | | |
$ | 504,788 | |
| Cost of revenue | |
| (620,000 | ) | |
| (614,000 | ) |
| Research and development | |
| (1,826,140 | ) | |
| (712,116 | ) |
| Selling, general and administrative | |
| (5,491,769 | ) | |
| (1,791,103 | ) |
| Other expense | |
| (1,765,962 | ) | |
| (1,683,322 | ) |
| Net Loss | |
$ | (9,603,871 | ) | |
$ | (4,295,753 | ) |
Revenue
Revenue
for the year ended March 31, 2020 and 2019 was $0.1 million and $0.5 million, respectively. The revenue recognized during the
year ended March 31, 2020 is related to our new agreement with Glass Manufacturer and represents the cash received for our continuing
development activities. The revenue recognized for the year ended March 31, 2019 is primarily comprised of $0.3 million of revenue
recognized with respect to our contract with Film Manufacturer and approximately $0.2 million of revenue recognized with respect
to our contract with g Glass Manufacturer. We are not able to estimate the total amount of development service under an efforts-based
perspective and, therefore, the amount of performance that will be required in our contracts cannot be reliably estimated under
the proportional performance revenue recognition model. Accordingly, we recognize revenue up to the amount of costs incurred.
Cost
of Revenue
The
cost of revenue for the year ended March 31, 2020 and 2019, was approximately $0.6 million for each period, respectively. The
cost of revenue for the year ended March 31, 2020 consists of approximately $0.5 million related to the costs incurred with respect
to our contract with Film Manufacturer and approximately $0.1 million with respect to our contract with Glass Manufacturer. The
cost of revenue for the year ended March 31, 2019 consists of approximately $0.4 million related to the costs incurred with respect
to our contract with Film Manufacturer and approximately $0.2 million with respect to our contract with Glass Manufacturer.
Research
and Development (including licenses acquired)
Research
and development expenses were $1.8 million for the year ended March 31, 2020 compared to $0.7 million for the year ended March
31, 2019. The increase of $1.1 million is primarily related to stock-based compensation expenses recognized for restricted stock
awards issued to our employees and officers.
Selling,
General and Administrative
SG&A
expenses were $5.5 million and $1.8 million for the years ended March 31, 2020 and 2019, respectively. The $3.7 million increase
in SG&A expenses was primarily attributable to increases in stock-based compensation expenses of $2.8 million recognized for
restricted stock awards issued to our employees and officers and for consulting services, and increases in professional fees of
$0.5 million primarily related to expenses incurred for our public offering.
Other
Income (Expense)
Other
expense was $1.8 million for the year ended March 31, 2020 compared with other expense of $1.7 million for the year ended March
31, 2019. The $0.1 million increase in other expense is primarily attributable to $0.4 million of increased interest expense related
to our convertible notes, $0.3 million recognized as a loss on extinguishment of debt related to the issuance of our common stock
in connection with our note amendments, offset by $0.6 million for the change in fair value of warrant liabilities related to
our convertible notes.
Liquidity
Going Concern
We have incurred substantial operating
losses since our inception, and we expect to continue to incur significant operating losses for the foreseeable future, and may
never become profitable. We had an accumulated deficit of approximately $16.4 million and $6.8 million at March 31, 2020 and 2019,
respectively, a net loss of approximately $9.6 million and $4.3 million, and approximately $1.0 million and $1.6 million of net
cash used in operating activities for the years ended March 31, 2020 and 2019, respectively.
We anticipate incurring additional losses
until such time, if ever, that we can obtain marketing approval to sell, and then generate significant sales, of our technology
that is currently in development. Substantial additional financing will be needed by the Company to fund our operations and to
develop and commercialize our technology. These factors raise substantial doubt about the Company’s ability to continue as
a going concern.
We will seek to obtain additional capital
through the sale of debt or equity financings or other arrangements to fund operations; however, there can be no assurance that
we will be able to raise needed capital under acceptable terms, if at all. The sale of additional equity may dilute existing stockholders
and newly issued shares may contain senior rights and preferences compared to currently outstanding shares of common stock. Issued
debt securities may contain covenants and limit our ability to pay dividends or make other distributions to stockholders.
Cash Flows
| | |
Years Ended March 31, | |
| | |
2020 | | |
2019 | |
| Cash and cash equivalents at the beginning of the period | |
$ | 99,447 | | |
$ | 168,222 | |
| Net cash used in operating activities | |
| (1,044,278 | ) | |
| (1,615,485 | ) |
| Net cash used in investing activities | |
| (26,603 | ) | |
| (109,290 | ) |
| Net cash provided by financing activities | |
| 1,019,741 | | |
| 1,656,000 | |
| Cash and cash equivalents at the end of the period | |
$ | 48,307 | | |
$ | 99,447 | |
Operating Activities
For the year ended March 31, 2020, net
cash used in operating activities was $1.0 million, which primarily consisted of our net loss of $9.6 million, adjusted for non-cash
expenses of $6.8 million including, $5.1 million of stock-based compensation expenses, $1.2 million of amortization related to
the debt discount recognized for our convertible notes payable, $0.3 million of loss on extinguishment of debt related to the issuance
of our common stock in connection with our note amendments and $0.2 million of expenses related to our public offering. The net
change in operating assets and liabilities was $1.7 million and was primarily due to increases in accounts payable and accrued
expenses totaling $1.3 million and increased accrued interest of $0.3 million related to our convertible notes.
For the year ended March 31, 2019, net
cash used in operating activities was $1.6 million, which primarily consisted of our net loss of $4.3 million, adjusted for non-cash
expenses of $2.7 million including, $1.0 million of stock-based compensation expenses, $1.0 million of amortization related to
the debt discount recognized for our convertible notes payable and $0.6 million for the change in fair value of our warrant liability.
The net change in operating assets and liabilities was nominal.
Investing Activities
For the year ended March 31, 2020, net
cash used in investing activities was approximately $27,000, related to the purchase of computer equipment and computer software.
For the year ended March 31, 2019, net
cash used in investing activities was $0.1 million, related to the purchase of computer equipment and computer software.
Financing Activities
For the year ended March 31, 2020, net
cash provided by financing activities was $1.0 million. The net cash provided is primarily related to $1.0 million of proceeds
received from the issuance of our senior secured convertible notes and the related stock warrants.
For the year ended March 31, 2019, net
cash provided by financing activities was $1.7 million. The net cash provided is primarily related to $1.8 million of proceeds
received from the issuance of our senior secured convertible notes and the related stock warrants, offset by a payment of $0.1
million related to our senior secured promissory note.
Off-balance sheet arrangements
We did not have any off-balance sheet arrangements
during the periods presented, and we do not currently have any off-balance sheet arrangements, as defined in the SEC rules and
regulations.
Revenue Recognition
We adopted the new revenue standard, ASC
606, on March 31, 2019 using the full retrospective approach. The adoption did not have an effect on 2020 or 2019 revenue recognition
or a cumulative effect on opening equity, as the timing and measurement of revenue recognition is materially the same as under
ASC 605. The core principle of the new revenue standard is that a company should recognize revenue to depict the transfer of promised
goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange
for those goods or services. The following five steps are applied to achieve that core principle:
| |
● |
Step 1: Identify the contract with the customer |
| |
● |
Step 2: Identify the performance obligations in the contract |
| |
● |
Step 3: Determine the transaction price |
| |
● |
Step 4: Allocate the transaction price to the performance obligations in the contract |
| |
● |
Step 5: Recognize revenue when the company satisfies a performance obligation |
For contracts where the period between
when we transfer a promised good or service to the customer and when the customer pays is one year or less, we have elected the
practical expedient to not adjust the promised amount of consideration for the effects of a significant financing component.
Our performance obligation is to provide
a development service that enhances an asset that the customer controls. We receive upfront payments in advance of providing services
and payment upon reaching milestones.
We are not be able to reasonably measure
the outcome of our performance obligations that are satisfied over time because we are in the early stages of the contracts. Therefore,
the amount of performance that will be required in our contracts cannot be reliably estimated and we recognize revenue up to the
amount of costs incurred.
Stock-based compensation
We measure and recognize compensation expense
for all options based on the estimated fair value of the award on the grant date. We use the Black-Scholes option-pricing model
to estimate the fair value of option awards. The fair value is recognized as expense on a straight-line basis over the requisite
service period. We account for forfeitures as they occur. We recognize expense for awards where vesting is subject to a market
or performance condition based on the derived service period. Expense for awards with performance conditions would be estimated
and adjusted on a quarterly basis based upon our assessment of the probability that the performance condition will be met.
The determination of the grant date fair
value of options using an option pricing model is affected principally by our estimated fair value of shares of our common stock
and requires management to make a number of other assumptions, including the expected life of the option, the volatility of the
underlying shares, the risk-free interest rate and expected dividends. The assumptions used in our Black-Scholes option-pricing
model represent management’s best estimates at the time of measurement. These estimates are complex, involve a number of
variables, uncertainties and assumptions and the application of management’s judgment, as they are inherently subjective.
If any assumptions change, our stock-based compensation expense could be materially different in the future.
These assumptions are estimated as follows:
| |
● |
Fair Value of Common Stock. As our common stock has not historically been publicly traded, we estimated the fair value of common stock. See “Fair Value of Common Stock” and “Common Stock Valuation Methodology” sections. |
| |
● |
Expected Term. The expected term represents the period that our options are expected to be outstanding. We calculated the expected term using the simplified method for options based on the average of each option’s vesting term and the contractual period during which the option can be exercised, which is typically 10 years following the date of grant. |
| |
● |
Expected Volatility. The expected volatility was based on the historical share volatility of several of our comparable publicly traded companies over a period of time equal to the expected term of the options, as we do not have any trading history to use the volatility of our own common stock. |
| |
● |
Risk-Free Interest Rate. The risk-free interest rate was based on the yields of U.S. Treasury securities with maturities appropriate for the term of the award. |
| |
● |
Expected Dividend Yield. We have not paid dividends on our common stock nor do we expect to pay dividends in the foreseeable future. |
Fair Value of Common Stock
Historically, for all periods prior to
this offering, the fair values of the shares of common stock underlying our options were estimated on each grant date by our board
of directors. In order to determine the fair value, our board of directors considered, among other things, contemporaneous valuations
of our common stock and preferred stock prepared by unrelated third-party valuation firms in accordance with the guidance provided
by the American Institute of Certified Public Accountants 2013 Practice Aid, Valuation of Privately-Held- Company Equity Securities
Issued as Compensation, or the Practice Aid. Given the absence of a public trading market of our capital stock, our board of directors
exercised reasonable judgment and considered a number of objective and subjective factors to determine the best estimate of the
fair value of our common stock, including:
| |
● |
contemporaneous third-party valuations of our common stock; |
| |
● |
the prices, rights, preferences and privileges of our preferred stock relative to our common stock; |
| |
● |
our business, financial condition and results of operations, including related industry trends affecting our operations; |
| |
● |
the likelihood of achieving a liquidity event, such as an initial public offering or sale of our company, given prevailing market conditions; |
| |
● |
the lack of marketability of our common stock; |
| |
● |
the market performance of comparable publicly traded companies; and |
| |
● |
U.S. and global economic and capital market conditions and outlook. |
Critical accounting policies and
significant judgments and estimates
Our financial statements are prepared in
accordance with generally accepted accounting principles in the United States, or GAAP. The preparation of our financial statements
requires us to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities, costs and expenses.
We base our estimates and assumptions on historical experience and other factors that we believe to be reasonable under the circumstances.
We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates. Our most critical
accounting policies are summarized below. See Note 3 to our condensed financial statements for a description of our other significant
accounting policies.
Recent accounting pronouncements
See Note 3 to our condensed financial statements
for a description of recent accounting pronouncements applicable to our financial statements.
JOBS Act Transition Period
As an “emerging growth company”
under the Jumpstart Our Business Startups Act of 2012, we can take advantage of an extended transition period for complying with
new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards
until those standards would otherwise apply to private companies. We are electing to delay our adoption of such new or revised
accounting standards. As a result of this election, our financial statements may not be comparable to the financial statements
of other public companies.
BUSINESS
Overview
Crown develops and sells optical switching
film that can be embedded between sheets of glass or applied to the surface of glass, or other rigid substrates such as acrylic,
to electronically control opacity (“DynamicTint™”). Developed under an exclusive license from HP, our technology
allows a transition between clear and dark in seconds and can be applied to a wide array of windows, including commercial buildings,
automotive sunroofs, and residential skylights and windows. At the core of Crown’s proprietary and patent-protected technology
is a thin film that is powered by electrically charged pigment which not only replaces common window tints but is also a more
sustainable alternative to traditional window treatments. Crown partners with leading glass and film manufacturers for mass production
and distribution of DynamicTint.
Electrokinetic Film Technology
Crown’s electrokinetic (EK) technology
was derived from proprietary ink and microfluidic technology developed at HP. Electrokinetic refers to the movement of particles
within a fluid under the influence of an electric field. Our EK film technology utilizes nanometer-sized pigment particles that
are electrically charged and suspended in a liquid that is sandwiched between two clear substrates that are coated with a transparent
conductor oxide (TCO) film. In a non-energized state, the suspended pigment particles are distributed uniformly between the plastic
films, and will absorb, transmit, or reflect light depending on the properties of the suspended pigment (dark state). When the
proper electrical signal is applied to the conductive TCO layers, an electrical field is created and the charged pigment particles
collect in micro-embossed holes in a layer of polymer resin covering the transparent conductor surface as shown in Figure 1.
As the charged pigment particles are collected, the fluid becomes highly transparent (light state). By applying a different electrical
signal, the pigment can be dispersed back into the fluid to achieve the desired color density or opaqueness.
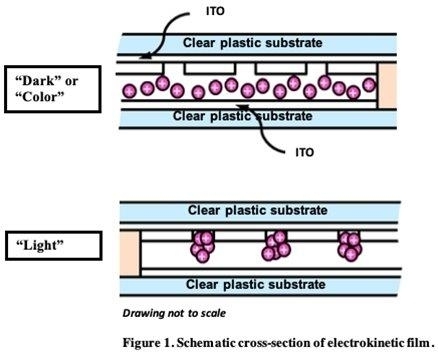
Our plastic films are manufactured using
industry standard roll-to-roll (R2R) processing equipment. The Company believes its R2R processing will have an inherently lower
manufacturing cost compared to sheet-based processing methods used for other smart window technologies like electrochromic glass.
There are three basic steps to making our film using R2R equipment.
| 1) | Deposition:
R2R TCO deposition on clear polyethylene terephthalate (PET) plastic film using vacuum sputtering of indium-tin oxide (ITO). The
ITO on PET film can be provided by a number of suppliers. Millions of square feet of ITO on PET are currently provided for nearly
all capacitance-based display touch screens. |
| 2) | Embossing:
R2R embossing of UV-curable resin in a proprietary 3-D pattern for ink pigment control and containment on one of the two plastic
films. This process is proprietary to Crown and protected by both Crown’s patents and trade secrets. An example of the embossed
pattern is shown in Figure 2. The R2R embossing process can be completed by various plastic film companies. Crown
has the capability to accomplish the deposition and embossing steps within its current facility. However, as production
increases, one of Crown’s future manufacturing partners will handle all production aspects of DynamicTint film. |

Figure 2. Microscopic Optical Image of
Embossed Film
| 3) |
Lamination: The final R2R process laminates the two layers of PET together with the pigment-containing fluid (which is proprietary to Crown and protected by patents and trade secrets) contained by the wall structure shown by the white areas in Figure 2. The wall area has adhesion to the upper layer of PET with ITO film thereby sealing the fluid between the two plastic layers. The fluid contains nanometer-sized pigment particles that are charged electrically and suspended in the fluid to keep the particles evenly distributed within the film and to minimize gravitational effects due to their small size and thermal motion of the pigment. Crown’s use of its pigment is proprietary and protected by its patents and trade secrets. |
We believe that DynamicTintTM has
the following six distinct advantages over existing optical electronic film technologies:
| |
● |
Neutral Color – Pigment is designed to be color neutral and will not affect the hue of what is viewed through the window in the dark or tinted state |
| |
|
|
| |
● |
Speed – Transition time is typically under 1-2 seconds |
| |
|
|
| |
● |
Affordability – Roll-to-Roll film manufacturing, inexpensive materials, and lower energy costs to operate DynamicTint |
| |
|
|
| |
● |
Low Energy Requirements – Film is low voltage and can be powered by solar strip, battery, or existing electrical infrastructure |
| |
|
|
| |
● |
Retro Fit – Film can be applied to a sheet of acrylic or heat-treated glass and attached to existing window frames, eliminating the need to replace single pane windows with dual pane windows. |
| |
|
|
| |
● |
Sustainable
– Reduces waste, energy consumption and uses renewable energy. |
Integration with Glass
Our electronic film can be cut using
standard laser cutting and then laminated between panes of glass for new window construction. DynamicTint will be laminated between
glass sheets for automotive applications or on a single glass sheet within an Insulated Glass Unit (IGU) (Figure 3). An
IGU typically consist of two or more panes of glass that are sealed along the edges with an inert glass like Argon between the
panes of glass to minimize heat transfer from one pane of glass to the other. Lower heat transfer improves insulating the outside
temperature from interior temperature. Power is provided to our device by two wires connected to a single small area on each ITO
surface. The wires will be routed through the IGU edge seal and can be connected to a control/power unit attached to the IGU for
individual window control. The electronics driving the film can be hardwired into the buildings HVAC control system or controlled
wirelessly depending on the customer’s needs. Because the overall power requirements are extremely low, localized batteries
in the control unit and/or in combination with a small area solar cell can be used to power DynamicTint.
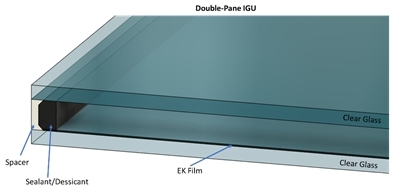
Figure 3. Double-paned IGU with EK Film
Retrofit with an Insert
DynamicTint can be laminated to other
surfaces like acrylic or heat-treated glass and the laminated sheet can be assembled in DynamicTint Inserts that can be placed
over the surface of existing windows (Figure 4).
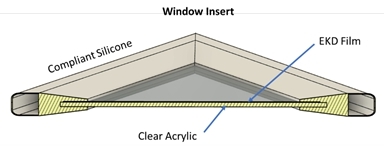
Figure 4. Window Insert with EK Film
We believe Crown’s DynamicTint
Inserts can be easily installed into residential skylights, residential windows as well as windows within garage doors. Additionally,
our DynamicTint Inserts can be used in commercial buildings to convert existing single pane windows into dual pane windows. Crown
believes that there is a significant opportunity to provide DynamicTint Inserts to commercial building owners who are looking
to eliminate window blinds, gain energy efficiency, and reduce carbon emissions.
Sustainability
Crown
is aware that working towards building a sustainable future is a common goal shared by many. Companies such as Walmart, Amazon
and Apple are now publishing their sustainability pledges, and we are seeing a trend of pledging to make their workplaces more
environmentally friendly.
Crown’s patented technology provides
a solution that helps address many sustainability issues such as:
| |
● |
Reducing waste – as opposed to replacing single pane window units with newly manufactured dual pane windows, Crown allows building owners to install our retrofit DynamicTint Insert into existing single pane window frames thereby creating a dual pane window; |
| |
● |
Reducing energy
– Crown’s DynamicTint Insert when installed in a single pane window frame, can reduce energy consumption used
by HVAC’s by reducing the need for constantly cooling and heating a room; and |
| |
● |
Using renewable
energy – Crown’s DynamicTint Insert can be powered by a solar cell that is integrated into the insert itself
thereby eliminating the need to hardwire the insert to the home or building’s electrical system. |
Crown’s
DynamicTint can reduce the amount of heat entering a building by controlling the tint of external windows. According to FacilitiesNet
(https://www.facilitiesnet.com/windowsexteriorwalls/article/Smart-Window-Benefit-Energy-Savings-Reduced-Glare--17280),
the ability to control the amount of heat entering a building reduces the heat load of the building which in turn reduces your
HVAC usage. Another benefit of DynamicTint is being able to optimize daylight usage, thereby reducing the usage of lights. A study
done by Project Drawdown (https://www.drawdown.org/solutions/dynamic-glass)
projected that if 30-50% of commercial building spaces install dynamic glass, the potential climate-weighted energy efficiency
from cooling is estimated at 9% and lighting at 9%—depending on local climate, building location and window orientation.
This can result in 0.3-0.5 gigatons of emissions reductions from decreased energy use.
At
Crown, we are committed to building a product that can be self-sufficient and does not require an additional power source or hard
wiring into the electrical system of a residential home or commercial building. While our DynamicTint helps keep light and heat
out of the building, we intend to harness that light and use it to power our inserts. This ensures that as we reduce a building’s
energy consumption, we are not adding to it and are working towards being carbon neutral.
Return on Investment
Crown calculates that for commercial
building owners, an average DynamicTint commercial building insert measuring five square feet would cost on average approximately
$600.00 per insert (not including installation). Using an average 500,000 square foot building with approximately 2,600 windows
that each measure five square feet, Crown estimates, based on internal research, that its insert will reduce a building owner’s
HVAC usage by up to 25% and would further eliminate the need to equip office windows with blinds. Based on the above factors,
Crown estimates energy savings of approximately $1.0 million over a ten-year period and a simple payback period of approximately
six years.
Crown further calculates that for commercial
building owners, purchasing DynamicTint as a film applied to a newly manufactured dual pane window (not including the cost of
the dual pane window) measuring five square feet would cost on average approximately $375.00 per window (not including installation).
Using an average 500,000 square foot building with approximately 2,600 windows that each measure five square feet, Crown estimates,
based on internal research, that its insert will reduce a building owner’s HVAC usage by up to 32%, or approximately $2.2
million in savings over a ten-year period and a simple payback period of approximately three years based on the same assumptions
outlined above.
Intellectual Property
On January 31, 2016, we entered into
an IP agreement with HP to acquire a research license to determine the feasibility of incorporating HP’s electro-kinetic
display technology in our products. Under the terms of the agreement, the license is to be used for research purposes only, has
a purchase price of $200,000 for the technology and has a two year closing date. On April 12, 2016, the Company and HP entered
into the first amendment to the agreement, which reduced the purchase price of the technology to $175,000, of which $75,000 was
payable upon completion of the technology transfer and $100,000 was payable upon the first anniversary of the agreement’s
effective date. The sales agreement entered into with HP concurrently with the first amendment to the agreement allocated $25,000
of the $200,000 purchase price to acquire equipment to be used in the research. On May 1, 2017, the Company and HP entered into
the second amendment to the agreement which increased the purchase price for the technology to $375,000 and extended the closing
date to January 31, 2020. Of such $375,000, $75,000 is payable upon completion of the technology transfer, $100,000 is payable
upon the first anniversary of the agreement’s effective date, $100,000 is payable upon the second anniversary of the agreement’s
effective date and $100,000 is payable upon the third anniversary of the agreement’s effective date. On March 10, 2019,
the Company and HP entered into the third amendment to the agreement, which extended the closing date to January 31, 2021, enumerated
certain intellectual property owned by HP that is not subject to the exclusive license granted to the Company and revised the
schedule of fees payable by the Company to HP, such that $100,000 is payable upon the first anniversary of the agreement’s
effective date, $100,000 is payable upon the second anniversary of the agreement’s effective date and $100,000 is payable
before April 20, 2019. As of January 8, 2021, the Company has paid $25,000 of the remaining $100,000 owed to HP for the research,
with the balance to be paid upon the exercise of the Company’s option to purchase the patents owned by HP by January 31,
2021. The agreement grants the Company an option to purchase the related assignable patents at a purchase price of $1.4 million.
In the event the Company exercises its option to purchase such patents, the Company will pay to HP a running royalty in the amount
of 3% of the gross revenues received by the Company and its affiliates for the sale, rental, license or other disposition of the
licensed products.
Since 2016, Crown has actively worked
to develop and license its EK technology, which it protects using patents, trade secrets and know-how. Crown currently licenses
from HP seven patents that have been issued in the United States, which the Company has the option to purchase prior to January
31, 2021 for a price of $1.4 million, subject to payment of the 3% royalty discussed above. Crown intends to exercise its option
to purchase such patents from HP on or prior to January 31, 2021. In addition, the Company has current patent applications in
the United States and other countries that if granted, would add three additional patents to its portfolio. The Company’s
United States patents expire at various dates from March 26, 2029 through September 26, 2032.
The Company believes that its EK technology
is adequately protected by its patent position and by its proprietary technological know-how. However, the validity of the Company’s
patents has never been contested in any litigation. The Company also possesses know-how and relies on trade secrets and nondisclosure
agreements to protect its technology. The Company requires any employee, consultant, or licensee having access to its confidential
information to execute an agreement whereby such person agrees to keep such information confidential.
Company-Owned Patents
| Application
No. |
|
Country |
|
Filing
Date |
|
Publication
No. |
|
Status |
|
Title |
| 16/259,078 |
|
USA |
|
January
28, 2019 |
|
20190256625 |
|
Pending |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| 201980018649.7 |
|
China |
|
January
28, 2019 |
|
CN111918894A |
|
Pending |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| 19704995 |
|
Europe |
|
January
28, 2019 |
|
3752867 |
|
Pending |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| 2020-566194 |
|
Japan |
|
January
28, 2019 |
|
|
|
Pending |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| 10-2020-7024977 |
|
Korea |
|
January
28, 2019 |
|
|
|
Pending |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| PCT/US2019/015464 |
|
WO |
|
January
28, 2019 |
|
WO
2019/160675 |
|
Expired |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| 62/631,623 |
|
USA |
|
February
16, 2018 |
|
|
|
Expired |
|
REFRACTIVE
INDEX MATCHED RESIN FOR ELECTROPHORETIC DISPLAYS AND OTHER APPLICATIONS |
| 16/741,622 |
|
USA |
|
January
13, 2020 |
|
2020-0225552 |
|
Pending |
|
APPLICATIONS
OF AN ELECTROKINETIC DEVICE FOR AN IMAGING SYSTEM |
| PCT/US2020/013396 |
|
WO |
|
January
13, 2020 |
|
WO2020/150166 |
|
Pending |
|
APPLICATIONS
OF AN ELECTROKINETIC DEVICE FOR AN IMAGING SYSTEM |
| 62/793,250 |
|
USA |
|
January
16, 2019 |
|
|
|
Expired |
|
APPLICATIONS
OF AN ELECTROKINETIC DEVICE FOR AN IMAGING SYSTEM |
| 15/552,924* |
|
USA |
|
August
23, 2017 |
|
10,852,615 |
|
Issued |
|
TWO
PARTICLE ELECTROPHORETIC LAMINATE FOR USE WITH SMART WINDOWS WITH REDUCED DIFFRACTION
|
| * | Co-owned with University
of Cincinnati |
In-Licensed Patents
| Patent
No. |
|
Country |
|
Patent
Date |
|
Status |
|
Title |
| 8,183,757 |
|
USA |
|
May
22, 2012 |
|
Issued |
|
DISPLAY
ELEMENT |
| 8,184,357 |
|
USA |
|
May
22, 2012 |
|
Issued |
|
DISPLAY
ELEMENT |
| 8,331,014 |
|
USA |
|
December
11, 2012 |
|
Issued |
|
PIGMENT-BASED
INKS |
| 8,384,659 |
|
USA |
|
February
26, 2013 |
|
Issued |
|
DISPLAY
ELEMENT INCLUDING ELECTRODES AND A FLUID WITH COLORANT PARTICLES |
| 8,432,598 |
|
USA |
|
April
30, 2013 |
|
Issued |
|
TRANSPARENT
CONDUCTOR STRUCTURE |
| 8,896,906 |
|
USA |
|
November
25, 2014 |
|
Issued |
|
INKS
INCLUDING BLOCK COPOLYMER GRAFTED PIGMENTS VIA AZIDE CHEMISTRY |
| 8,018,642 |
|
USA |
|
September
13, 2011 |
|
Issued |
|
ELECTRO-OPTICAL
DISPLAY |
Business Model
We intend to develop and sell our patented
EK Technology under the name DynamicTintTM. We intend to generate revenue by selling, and in some cases licensing, DynamicTintTM
film and other technical know-how to our customers and licensees that integrate the technology into their products. We are in discussions
with multiple glass manufacturers, window manufacturers, film manufacturers, and building owners to both produce, distribute and
buy DynamicTintTM film.
Applications we are exploring with potential
customers of Crown’s DynamicTint include:
| |
● |
Automotive: sunroofs and sun visors; |
| |
● |
Aerospace, rail and marine: windows, partitions, sun visors, and skylights; |
| |
● |
Residential homes: residential windows, garage door windows, windows contained in and surrounding residential front doors as well as residential skylights; and |
| |
|
|
| |
● |
Commercial and multi-family buildings: external windows, internal glass walls and doors for both new construction and retrofit. |
Crown’s first product will be
the DynamicTint Insert for residential skylights. Crown has developed a working prototype of an insert for the residential skylight,
which allows a homeowner to control the amount of light entering the room. Crown’s patented DynamicTint Insert does not
require the homeowner to replace their skylight as it conveniently snaps into the existing frame without the need for fasteners
or any tools and can be easily installed in a matter of minutes. Usually positioned high in a home’s ceiling and out of
reach, the vast majority of existing skylights cannot prevent unwanted glare, light, or heat. Crown’s skylight insert, however,
will allow a homeowner (through a Blue Tooth connection or RF controller) to easily and quickly adjust the level of desired tint,
thereby controlling the amount of light (and subsequently heat) entering the room. The DynamicTint Skylight Insert can be powered
by either a solar cell or battery backup thereby eliminating the need to hardwire the insert to the home’s electrical system.
Utilizing the same insert strategy
as described above for existing residential skylights, Crown is in active discussions with Hudson Pacific Properties (NYSE: HPP)
(who is also a strategic investor in Crown) about deploying our DynamicTintTM film as a retrofit insert product on
their existing external glass for a majority of the 108 buildings within their portfolio. Crown’s Commercial Building Insert
would allow the building owner to quickly convert its single pane window units to a dual pane window unit. Crown’s insert
would act as the “second pane” and would allow the building owner to enjoy all the benefits of a dual pane window
without having to replace their existing single pane windows. Crown’s insert can be integrated into the building HVAC control
system, thereby optimizing the use of our DynamicTint Insert and reducing the use of the HVAC to heat or cool the rooms utilizing
our technology. As Crown’s DynamicTint technology requires very little energy to effect that transition from clear to dark
state, a solar cell coupled with a battery backup eliminates the need to hardwire the inserts to the building electrical system.
Crown believes that the potential retrofit market for its DynamicTint Building Inserts is significantly large.
Crown’s commercialization strategies
are deeply rooted in leveraging existing infrastructure. As such, Crown intends to partner with industry leading manufacturers
of glass and windows as well as manufacturers of plastic film who have roll-to-roll capabilities to efficiently and inexpensively
produce our film. While Crown will develop and own certain tooling that allows its film to be produced on a roll-to-roll film manufacturing
line, Crown will leverage the existing infrastructure of partner manufacturers to enable Crown to avoid the extensive capital costs
of building its own manufacturing facilities. In addition, Crown believes that it can also leverage its manufacturing partner’s
existing sales and distribution channels.
As Crown moves from its research and development
stage and into its first commercialization stage, it has engaged with numerous existing and experienced thin film manufacturers
about collaborating in the mass production of DynamicTint. Those ongoing discussions in combination with the ongoing development
work with two of Crown’s existing manufacturing partners, is expected to allow Crown to move from a “development only”
stage into commercialization stage in 2021.
Partners and Customers
Crown is in active discussions with multiple
glass and film manufacturers for assessment of its DynamicTint technology and its application to glass markets around the world.
Those ongoing discussions have led to two agreements in which Crown is being paid development revenue to provide samples and prototypes
for specific markets.
On November 15, 2017, the Company entered
into a license agreement with the Glass Manufacturer. This agreement provides that the Company will provide samples to be used
by the Glass Manufacturer for the sole purpose of determining the feasibility of integrating the DynamicTint technology in the
Glass Manufacturer’s auto and train glass products, as well as potentially commercial and residential glass and windows.
The Company began performing development activities in April of 2018. On February 1, 2019, the Company and the Glass Manufacturer
entered into a new license agreement, terminating the prior agreement, which was further extended on November 14, 2019. Under
such new license agreement, the Company will provide samples to be used by the Glass Manufacturer to evaluate the appearance of
and measure optical properties of the Company’s film technology. At the Glass Manufacturer’s option, the Company will
provide additional samples to be used by the Glass Manufacturer to measure the durability of such sample for the purpose of determining
the feasibility of integrating the Company’s film technology in the Glass Manufacturer’s auto and train glass products.
The performance related to the new agreement is a continuation of the work being performed as of April 2018. Crown has made significant
improvements to its contrast ratio, haze, uniformity, switching speed, lamination capabilities, and optical defects among other
technical and visual improvements. These improvements have allowed Crown to move from the development stage to the production/commercialization
stage.
On August 23, 2017, the Company entered
into a collaborative agreement with the Film Manufacturer. The Film Manufacturer agreement provides that the Company and Film
Manufacturer will jointly develop electrokinetic films and determine their suitability for commercial use in applied films and
interlayers for application to aftermarkets automobile sunroofs and windows. The Company began performing development activities
in April of 2018 and is expected to continue the relationship and expand into commercialization of DynamicTint in 2021.
Smart Glass Industry Trends
We believe there are favorable converging
global trends in the major near-term markets for “smart glass” products. Key factors driving the growth of the smart
glass market are the growing demand for smart glass in automobile applications, government support through mandates and legislation
for energy-efficient construction, and energy savings through smart glass applications.
In both public and private sectors across
the world, there are substantial efforts targeted toward the promotion and use of energy efficient smart glass materials, including
those used in automobiles, windows and other architectural glazings.
In September 2020, Markets and Markets
issued Smart Glass Market with COVID-19 Impact by Technology (Suspended Particle Display, Electrochromic, Liquid Crystal),
Application (Architecture, Transportation, Consumer Electronics), and Geography - Global Forecast to 2025. The smart glass
market size is expected to grow from USD 3.8 billion in 2020 to USD 6.8 billion by 2025, at a CAGR of 12.1% during the forecast
period. The growth of the smart glass industry is driven by factors, such as the growing adoption of smart glass in automotive
application and, declining prices for electrochromic material. Other major driving factors for smart glass adoption
include supportive government mandates and legislation on energy efficiency. Governing bodies of various countries are increasingly
encouraging the use of these energy-efficient products. Smart glass has inherent energy-saving and auto-dimming properties, which
reduce its maintenance cost. As a result, the perceived benefits of these glass products are more than the incurred investments.
Crown believes that the smart glass industry
is in the initial phase of growth and that DynamicTintTM may have commercial applicability in many products where variable
light-control is desired.
Our Technology
DynamicTintTM combines many
of the favorable properties of the other smart window technologies, which are described below. It has a fast switching time (1-2
sec.) like Suspended Particles in Polymer (SPD) and Polymer Dispersed Liquid Crystal (PDLC) technology, but does not need alternating
current power, requires less power and can tint to black. Unlike electrochromic (EC) technology, modulation in light level is not
area dependent, has much faster switching speeds, can tint to black and uses direct current pulses to change state quickly, allowing
for much lower power consumption. DynamicTint is also expected to have good bi-stability, so that when a light level of the film
is selected, the film will remain unchanged for extended time periods with little to no electrical power required. Because of the
low power requirements, DynamicTint can be powered with batteries or small area solar cells, allowing retrofit to existing windows
with ease and without the need to hard wire to existing electrical systems.
There are also major differences resulting
from the fact that different color nanoparticles can be used in DynamicTintTM whereas competing technologies are either
black, gray or blue tinted. Furthermore, with DynamicTint it is possible to use multiple colorants in the same film, which has
been demonstrated in the recent past under a research project at the University of Cincinnati.
Other Smart Glass Technologies
Variable light transmission technologies
can be classified into two basic types: “active” technologies that can be controlled electrically by the user either
automatically or manually, and “passive” technologies that can only react to ambient environmental conditions such
as changes in lighting or temperature. Most of the technologies are “active”. One type that is passive is thermochromic
technology where a rise in temperature will darken the film applied to glass.
The Company believes that our DynamicTint
has certain performance advantages over other “smart glass” technologies and that pricing and product performance
are the two main factors critical to the adoption of smart glass products. Because the non-EK smart glass technologies listed
below do not have published, consistent pricing or cost data that can be relied upon, the Company cannot accurately report its
price position relative to these other technologies. In terms of product performance, the Company believes that DynamicTint offers
numerous advantages over other smart glass technologies as discussed below.
| Technology |
|
Can
Retrofit |
|
Power
Usage |
|
Can
Tint to Black |
|
Solar
or Battery Powered |
|
Tint
Transition Speed |
|
Light
Transmission |
| DynamicTintTM
(Electrokinetic) |
|
|
|
<0.01
W/M2 |
|
✓ |
|
✓ |
|
~2
sec |
|
1.0%
- 70% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Electrochromic
(EC) |
|
✕ |
|
0.3 – 2 W/M2
(30X EK) |
|
✕ |
|
✕ |
|
5-40
min |
|
1%
- 58% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Suspended
Polymers in Particles (SPD) |
|
✕ |
|
~1.3 W/M2
(130X EK) |
|
✕ |
|
✕ |
|
3
- 5 sec |
|
3%
- 62% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Polymer
Dispersed Liquid Crystal (PDLC) |
|
✕ |
|
5 – 20 W/M2
(500X EK) |
|
✕ |
|
✕ |
|
1
– 3 sec |
|
~80%
* Does not block light |
Electrochromic
Glass
Electrochromic (EC) glass technology has
been used as a light absorbing technology for rear view mirrors in automobiles for decades, and more recently for large-scale windows.
However, the EC technology developed for windows is based on a different set of materials that are directly deposited on the heat-treated
glass panels. All the current EC companies are using tungsten oxide as the main component involved in the color transition from
clear to blue. Because of the nature of the chemical transition of the tungsten oxide, the EC film does not absorb as much of the
blue light and so remaining light will have a strong blue hue both in the room and looking through the window. The speed of the
switching from dark to light or the reverse change is directly related to the size of the window area and the electrode design
which brings electrical current to the EC material to start the chemical transition. EC technology is basically a battery-like
material that requires “charging and discharging”. The time to charge/discharge the EC material in a large window can
take up to 40 minutes to change form the dark state to the clear state at nominal temperatures. Also, during switching of the EC
film, there can be non-uniform areas which can vary in level of tint from center to edge. The larger the area of the window, the
more non-uniform during the change of state. Longer switching time can minimize the non-uniform areas. The EC materials are typically
vacuum deposited directly on “defect-free” glass. The typical investment required for a large window electrochromic
factory can run into the hundreds of millions of dollars, due to the large-scale vacuum equipment required, low particulate cleanroom
required, and the relatively slow speed of deposition for all the various layers. Kinestral Technologies is using a chemical liquid
deposition technique to replace some of the vacuum deposition steps to lower the capital investment needed for manufacturing.
Suspended Particle Glass (SPD)
SPD is a film that has suspended long
and narrow particles in an encapsulated liquid polymer film with layers of ITO on either side to allow generation of an alternating
current electrical field to twist the particles from a random state to a near vertical state perpendicular to the ITO plane. In
the vertical state light passes through the film and in the random state the light is absorbed by the particles. The color of
the film is blue since the particles used in the film don’t absorb blue light as well as other colors of sunlight. No other
types of particles have been created for this type of device. The film responds quickly to the electrical field, however, requires
constant high AC voltage to hold the clear state. The film is manufactured on plastic and uses roll-to-roll (R2R) equipment processing.
Also, because the particles are aligned when in the clear state, the film has a limited viewing angle much like older liquid-crystal
displays. When viewed at a side angle, the film will appear darker. The current market for SPD has been mainly automobile sunroofs
where the viewing angle of the passengers is relatively fixed at nearly perpendicular angle to the SPD film.
Polymer-Dispersed Liquid Crystal (PDLC)
Film
PDLC requires an AC electric field like
the SPD film described above to achieve a clear state. However, the liquid-crystal based film can only scatter light in the power-off
state, therefore, most of the incoming light is transmitted through the film (~80%). Typically, the PDLC film is used for interior
windows or doors to create privacy. PDLC has similar manufacturing methods using R2R equipment and plastic film with ITO conductor
to the SPD film. The film is available from many Far East manufacturing companies with some able to make ~150 cm width film. The
quality of the film can vary based on the manufacturing company. The film was invented at Kent State University in the 1980’s
and the patents have expired.
Competition
Several smart glass competitors have
an operating history, including:
| |
● |
SAGE Electrochromics, Inc., a wholly owned subsidiary of Saint-Gobain, which develops and manufactures electrochromic glass; |
| |
● |
View Glass and Kinestral Technologies manufacture electrochromic glass at their purpose-built manufacturing facilities and both are headquartered in California; and |
| |
● |
Research Frontiers, Inc. licenses an electronically controlled tinted film, utilizing SPD technology, to various companies. |
Crown Electrokinetics expects that other competitors will emerge
in the future.
Research and Development
Crown has been using a 6” width R2R
equipment capable of handling the deposition, embossing and lamination steps of the manufacturing process for its research and
development for the past three years and Crown will have its proto-manufacturing roll-to-roll equipment at 12” width available
in early 2021. Production prototypes for qualification and system testing will be sourced from the 12” equipment in 2021.
Crown will utilize the 12” width film for the DynamicTint Skylight Insert. Larger scale manufacturing is planned at a minimum
of 24” width film to address markets including larger format skylights inserts, appropriately sized residential and commercial
building window inserts, and many automobile sunroofs beginning in mid-to-late 2021. Thereafter, one of Crown’s future manufacturing
partners will follow with a roll-to-roll line to accomplish all necessary steps to manufacture DynamicTint of at least 60”
width capability. This will allow Crown to address the vast majority of window sizes for all applications.
As a result of the Company’s research
and development efforts, the Company believes that its EK technology, in some cases, is now, or with additional development will
become, usable in a number of commercial products. Such products may include one or more of the following fields: “smart”
windows, doors, skylights and partitions; self-dimmable automotive sunroofs, windows, sun visors, and mirrors.
The Company has devoted most of its financial
resources to research and development activities with the goal of producing commercially viable EK products and has developed working
samples of its EK technology.
Crown’s main goals in its research
and development include:
| |
● |
developing wider ranges of light transmission and quicker switching speeds, |
| |
● |
developing different colored version of Crown’s DynamicTint, |
| |
● |
reducing the voltage required to operate EK samples, |
| |
● |
obtaining data and developing improved materials regarding environmental stability and longevity, and |
| |
● |
quantifying the degree of energy savings expected by users of the Company’s technology including the degree that EK technology can control heat and its contribution to energy savings directly and through daylight harvesting strategies in sustainable building designs. |
Subsequent Events
On November 13, 2020, the Company entered
into a Securities Purchase Agreement with certain institutional and accredited investors to sell to the investors senior convertible
debentures, convertible into the Company’s common stock at a conversion price of $3.75 per share, subject to adjustment
as set forth in the debenture. The debentures have a maturity of one year, accrue interest at the rate of 7% per year, and are
subject to 12.5% original issue discount. Each investor also received a warrant to purchase a number of share of the Company’s
common stock equal to 50% of the number of shares the debenture is convertible into. The warrants have a five-year term, and an
exercise price of $4.50 per share, subject to adjustment as set forth therein.
The Crown Electrokinetics Corp. 2020
Employee Incentive Plan (the “2020 Plan”) was adopted by the Board of Directors and approved by our stockholders on
December 24, 2020. The 2020 Plan permits the following types of awards: stock options, stock appreciation rights, restricted shares,
performance units and other types of awards. The Company reserved 5,333,333 shares of common stock for issuance under the 2020
Plan. As of today’s date, the Company has not granted any stock awards under the 2020 Plan. The 2020 Plan will terminate
on December 24, 2030.
On January 4, 2021, the Company cancelled
2,000,000 restricted stock awards previously granted to employees of the Company.
On January 5, 2021, the Company authorized
the creation of the Company’s Series A Preferred Stock (the “Series A Preferred Stock”). A holder of convertible
promissory notes issued by the Company have agreed to convert an aggregate of $343,423.02 of debt pursuant to such notes into
360,111 shares common stock and 251 shares of Series A Preferred Stock simultaneously with the closing of this offering. The holder
will also receive warrants to purchase 235,183 shares of common stock at an exercise price of $0.13 per share and 88,871 shares
of common stock at an exercise price of $1.13 per share.
On January 11, 2021, the Company authorized
the creation of the Company’s Series B Preferred Stock (the “Series B Preferred Stock”). A holder of convertible
promissory notes issued by the Company have agreed to convert an aggregate of $1,496,751.82 of debt pursuant to such notes into
173,111 shares of common stock and 1,443.41 shares of Series B Preferred Stock simultaneously with the closing of this offering.
The holder will also receive warrants to purchase 940,730 shares of common stock at an exercise price of $0.13 per share and 355,485
shares of common stock at an exercise price of $1.13 per share.
Certain other holders of the Company’s
convertible promissory notes have agreed to convert their convertible promissory notes in an aggregate principal amount of $749,660
into 199,910 shares of common stock at a conversion price of $3.75 per share simultaneously with the closing of this offering.
Employees
The Company has eleven full-time employees
and five advisors. Seven of the employees are technical personnel, and the rest perform business development, legal, finance,
marketing, investor relations, and administrative functions. Of these employees, three have obtained doctorates, one has a master’s
degree in chemistry, and one has extensive industrial experience in electronics and electrical engineering. Two employees also
have additional postgraduate degrees in business administration, and one has a doctorate in jurisprudence. Also, the Company’s
suppliers and licensees have well qualified personnel on their teams with advanced degrees in a number of areas relevant to the
commercial development of products using the Company’s technology. The success of the Company is dependent upon, among other
things, the services of its senior management, the loss of which could have a material adverse effect upon the prospects of the
Company. None of our employees are represented by a labor union or covered by a collective bargaining agreement.
As Crown continues to grow, we will add
additional engineering, marketing and executive level personnel.
Legal Proceedings
In August 2019, Spencer Clarke LLC (“Spencer
Clarke”) filed a lawsuit against Crown in the Supreme Court of the State of New York, County of New York, Index No. 654592/2019.
Spencer Clarke has asserted claims arising from a 2018 Placement Agent Agreement (the “Placement Agent Agreement”)
under which Spencer Clarke agreed to assist Crown in raising money for a potential public offering. Spencer Clarke claims that
Crown failed to make certain payments under that Placement Agent Agreement. On September 27, 2019, Crown filed a motion to dismiss
the complaint. On October 7, 2019, Spencer Clarke amended the complaint. On November 8, 2019, Crown filed an Answer and asserted
Counterclaims against Spencer Clarke alleging breach of contract, anticipatory repudiation, and tortious interference with prospective
business relations. Crown disputes that it owes any money to Spencer Clarke and is vigorously defending the claims against it.
Available Information:
Crown Electrokinetics is located at 1110
NE Circle Blvd, Corvallis, OR 97330. Our telephone number is +1 (800) 674-3612 and our Internet website address is www.crownek.com.
MANAGEMENT
Management and Board of Directors
Our current members of the Board of Directors
and executive officers are listed below.
| Name |
|
Age |
|
Title |
| Executive
Officers |
|
|
|
|
| Douglas Croxall
|
|
51 |
|
Chairman &
Chief Executive Officer |
| Tim Koch |
|
59 |
|
Chief Technology
Officer |
| Phil Anderson |
|
53 |
|
Chief Financial
Officer |
| Non-Employee
Directors |
|
|
|
|
| Dr. DJ Nag |
|
50 |
|
Director |
| Edward Kovalik |
|
46 |
|
Director |
| John Marchese |
|
69 |
|
Director |
| Christopher Smith |
|
82 |
|
Director |
All directors serve for one year and until
their successors are elected and qualified. All officers serve at the pleasure of the Board of Directors. There are no family relationships
among any of our officers and directors.
Information concerning our executive officers
and directors is set forth below.
Douglas Croxall. Mr. Croxall is
the Chief Executive Officer and Chairman of the Board of Directors of Crown Electrokinetics Corp. Prior to co-founding Crown Electrokinetics,
Mr. Croxall was the CEO and Chairman of the Board of Directors of Marathon Patent Group from November 2012 until December 2017.
Mr. Croxall holds a BA degree from Purdue University and an MBA from Pepperdine University.
Timothy Koch. Mr. Koch is the Chief
Technology Officer of Crown Electrokinetics. Prior to co-founding Crown, he was in charge of the R&D team at HP that invented
electrokinetic (EK) technology. He has over 30 years of engineering and management experience in both technology development and
product manufacturing. He holds a BS from Cornell University and a MS from Stanford University, both degrees in Material Science
& Engineering. He has also completed an Executive Development Program from the Cornell University Johnson Graduate School of
Management.
Phil Anderson. Mr. Anderson is the
Chief Financial Officer of Crown Electrokinetics. Prior to his appointment as the Company’s CFO, from June 2019 to January
2020, Mr. Anderson served as a consultant for Kubient Inc. At Kubient, Mr. Anderson helped guide Kubient through its initial public
offering process. From June 2017 to May 2019, Mr. Anderson served as the Chief Financial Officer of Edison Nation Inc. (NASDAQ:
EDNT), where he was responsible for maintenance and preparation the company’s financial statements, as well as the company’s
initial public offering process. Prior to Edison Nation Inc., Mr. Anderson was the Chief Financial Officer of Electronic Cigarettes
International Group Ltd. (OTCBB: ECIG) from January 2015 through May 2017. In that role, Mr. Anderson coordinated the restructuring
of the company’s debt and multiple issuances of senior secured loans. Mr. Anderson was also responsible for coordinating
the company’s public company filing obligations. Earlier in his career, Mr. Anderson gained significant equity research experience.
Non-Employee Directors
Dr. DJ Nag has served as a member
of our board of directors since July 2020. Dr. Nag is the Chief Investment Officer at Ventech Solutions, a healthcare technology
company that manages quality data for the Center for Medicare and Medicaid Services (CMS). He has successfully led Ohio State University,
Rutgers University and University of Nebraska’s technology transfer operations that included licensing, startup and investments.
As an entrepreneur, he led a number of start-ups in the intellectual property strategy, artificial intelligence, and medical device
space. As a consultant in patent monetization and intellectual property strategy, he has worked with many Fortune 500 companies,
universities, and national governments. He was a Director of Ocean Tomo and a Vice President at ICAP Ocean Tomo, leading patent
transaction markets. He was recognized as one of the top IP strategists by IAM300 in 2019. Dr. Nag was on the Board of the Association
of University Technology Managers, Inc. (AUTM) from 2012 to 14, focused on educating the members around world on the importance
of technology transfer and intellectual property. He is widely recognized as a global intellectual property strategist working
with government and universities in Poland, Japan, India, Turkey, Brazil, South Korea, Ukraine and many other countries. Currently,
he teaches intellectual property strategy and negotiations as a Professor of Practice at Rutgers University and a Visiting Professor
at Shizuoka University. He volunteers as the first Executive-in-Residence at the Dublin City Schools, leading a startup academy
for high school students and serves on the foundation board at the Dublin Methodist Hospital.
Edward Kovalik has served as
a member of our board of directors since December 2020. Mr. Kovalik is the Chief Executive Officer of Unity National Financial
Services, a minority owned boutique investment bank. He is also a co-Founder of Prairie Partners, a renewable energy investor
in utility-scale solar and wind projects. Prior to Unity National, Mr. Kovalik was the co-Founder and, from April 2012 through
October 2020, the Chief Executive Officer of KLR Group, a merchant bank focused on the Energy sector. Mr. Kovalik also served
as the Chief Executive Officer of Seawolf and the President of KLR Energy Group, a special purpose acquisition company, both of
which were portfolio companies of KRL. His expertise includes private and public offerings of debt and equity, M&A, and fund
management. While at KLR, Mr. Kovalik led the creation of Rosehill Resources an independent Oil & Gas Company created through
a merger of KLR’s SPAC with Tema Oil & Gas. Mr. Kovalik also led the creation of Seawolf Water, a premier provider of
water solutions to the Oil & Gas industry, for which he also served as CEO. Prior to KLR, Mr. Kovalik served as the Head of
Capital Markets at Rodman & Renshaw, the highest ranked PIPEs practice in the US from 2005-2011. He has served on multiple
private and public boards of directors and is a member of NACD, the National Association of Corporate Directors.
John Marchese has been a member
of our board of directors since December 2020. In June 2000, he founded Marchese Associates, a branding and integrated marketing
consultancy located in Jacksonville Beach, Florida. He has served as the firm’s president and managing partner since its
founding. With over 35 years of marketing and brand consulting experience, Mr. Marchese has worked with C-level executives of
Fortune 500 companies to solve highly-complex business and portfolio marketing problems. Areas of B2B, B2C and D2C experience
include enterprise technologies and software, consumer products, financial services, pharmaceuticals, automotive, retail, and
digital services.
Prior to Marchese Associates, Mr. Marchese
served concurrently as President of the Americas for Bates Worldwide (an advertising agency), and CEO of the America’s for
141 Worldwide (an integrated marketing company) within Cordiant Communications Group plc. Prior to Cordiant, he was President
of Omnicom Group’s Alcone Marketing Group, an integrated marketing agency, and managing director of Wieden + Kennedy Advertising.
Mr. Marchese holds a Bachelor of Arts degree from Fordham University, and has been an active participant in the Harvard Business
School’s Executive Training Program.
Christopher Smith has been a
member of our board of directors since December 2020. Over the past five years, Mr. Smith has practiced corporate and personal
law for a number of private individuals, family offices and corporations, in addition to serving as the Managing Principal of
his legal and financial advisory firm, Alexander Smith & Company, Inc. From 2018 through June 2020, Mr. Smith served as Director,
Chairman of the Audit Committee and General Counsel of First Coast Security, a national provider of security services to data
centers and corporate installations, and as Manager of Mistral America’s LLC, a privately held real estate investment firm.
Since July 2019, Mr. Smith has been Lead Director, Chairman of the Governance Committee and member of the Audit Committee for
Kubient, Inc. (NASDAQ: KBNT), an advertising technology company. He has also served as either Director, Executive Chairman, Chief
Executive Officer, General Counsel or Chief Financial Officer of Oneida, Ltd., Sylvania International, Cadence International,
Atkins Nutritionals, Puma (USA), Barnes Engineering, London Fog, Escada AG and the Bronx Zoo. When previously serving as a Board
member, Mr. Smith has chaired both Audit and Governance committees. Mr. Smith is a graduate of Williams College and the Yale Law
School. He is admitted to the bar in New York, Connecticut and the District of Columbia and continues to be active in the practice
of law.
Family Relationships
There are no family relationships between
any of our executive officers and directors.
Board of Directors
Each of our directors will be elected at
our annual meeting of stockholders and hold office until the next annual meeting of stockholders, or until a successor is elected
and qualified. If any director resigns, dies or is otherwise unable to serve out the director’s term, or if the Board increases
the number of directors, the Board may fill the vacancy by the vote of a majority of the directors then in office. A director elected
to fill a vacancy shall serve for the unexpired term of such director’s predecessor.
Director Independence
We have applied to have our common stock
listed on the Exchange, subject to notice of issuance under the trading symbol “CRKN”. The Exchange Listing Rules require
that independent directors compose a majority of a listed company’s board of directors within one year of listing. In addition,
the Exchange Listing Rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation,
and nominating and corporate governance committees be independent and that audit committee members also satisfy independence criteria
set forth in Rule 10A-3 under the Exchange Act of 1934. Under the Exchange Listing Rules, a director will only qualify as an “independent
director” if, in the opinion of our board of directors, that person does not have a relationship that would interfere with
the exercise of independent judgment in carrying out the responsibilities of a director. In order to be considered independent
for purposes of Rule 10A-3 under the Exchange Act, a member of an audit committee of a listed company may not, other than in his
or her capacity as a member of the audit committee, the board of directors, or any other board committee: (1) accept, directly
or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or (2) be
an affiliated person of the listed company or any of its subsidiaries.
In addition, members of the compensation
committee must satisfy additional independence requirements set forth in the Exchange Listing Rules. In order to be considered
independent for purposes of the Exchange Listing Rules, a member of a compensation committee of a listed company may not, other
than in his or her capacity as a member of the compensation committee, the board of directors, or any other board committee, accept,
directly or indirectly any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries.
Additionally, the board of directors of the listed company must consider whether the compensation committee member is an affiliated
person of the listed company or any of its subsidiaries and if so, must determine whether such affiliation would impair the director’s
judgment as a member of the compensation committee.
Our board of directors undertook a
review of its composition, the composition of its committees and the independence of each director. Based upon information requested
from and provided by each director concerning his background, employment and affiliations, including family relationships, our
board of directors determined that Messrs. Nag, Kovalik, Marchese and Smith do not have any relationships that would interfere
with the exercise of independent judgment in carrying out the responsibilities of a director and that each is considered an “independent”
director as that term is defined under the applicable SEC rules and the Exchange Listing Rules. In making those determinations,
our board of directors considered the current and prior relationships that each non-employee director has with our Company and
all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial
ownership of our capital stock by each non-employee director.
Committees of the Board of Directors
We have an Audit Committee, Compensation
Committee, and Governance and Nominating Committee. Our Audit Committee consists of three independent directors who are Mr. Marchese,
Mr. Kovalik and Mr. Smith, with Mr. Kovalik considered as an “audit committee financial expert” within the meaning
of Regulation S-K of the SEC. Our Compensation Committee consists of three independent directors who are Dr. Nag, Mr. Kovalik
and Mr. Marchese. Our Governance and Nominating Committee consists of three independent directors who are Dr. Nag, Mr. Marchese
and Mr. Smith.
Executive Compensation
Compensation Table
| | |
| | |
| | |
| | |
| | |
Long-Term
Compensation Awards
| |
| | |
Annual
Compensation | | |
Other | | |
| | |
Restricted
Stock | |
| Name and Principal Position | |
Fiscal
Year | | |
Salary | | |
Bonus | | |
Compensation | | |
Options | | |
Awards | |
| Douglas Croxall | |
| 2020 | | |
$ | - | | |
$ | - | | |
$ | 310,000 | (1) | |
$ | - | | |
$ | 2,050,000 | |
| Chief
Executive Officer | |
| 2019 | | |
$ | - | | |
$ | - | | |
$ | 250,000 | (1) | |
$ | 241,253 | | |
$ | - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| James Douvikas | |
| 2020 | | |
$ | 178,542 | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 656,000 | |
| Former
Chief Executive Officer (current Manager of Business Development) | |
| 2019 | | |
$ | 209,951 | | |
$ | - | | |
$ | - | | |
$ | 48,251 | | |
$ | - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Timothy Koch | |
| 2020 | | |
$ | 180,000 | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 656,000 | |
| Chief
Technology Officer | |
| 2019 | | |
$ | 202,539 | | |
$ | - | | |
$ | - | | |
$ | 48,251 | | |
$ | - | |
| (1) | The
other compensation paid to Mr. Croxall relates to consulting fee payments. |
Stock Option Grants
A total of 2,960,000 restricted stock
units have been issued to employees, and 198,333 restricted stock units have been granted to advisors.
A total of 1,646,166 stock options have been granted to
employees, 291,666 stock options have been granted to advisors.
Outstanding Equity Awards at Fiscal
Year End
2020 Outstanding Equity Awards at Fiscal
Year-end Table
The following table sets forth information
regarding the outstanding equity awards held by our Named Executive Officers as of the end of our 2020 fiscal year:
| | |
Option
Awards | |
Stock
Awards | |
| Name | |
Number
of Securities Underlying Unexercised Options
(#)
Exercisable | | |
Number
of Securities Underlying Unexercised Options
(#)
Unexercisable | | |
Equity
Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options
(#) | | |
Option
Exercise Price
($) | | |
Option
Expiration
Date | |
Number
of Shares or Units of Stock That Have Not Vested
(#) | | |
Market
Value of Shares or Units of Stock That Have Not Vested
($) | | |
Equity
Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested
(#) | | |
Equity
Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested
($) | |
| Douglas
Croxall | |
| 86,806 | | |
| 79,860 | | |
| 166,666 | | |
$ | 0.15 | | |
February 28, 2028 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 145,833 | | |
| 104,166 | | |
| 250,000 | | |
$ | 1.20 | | |
January 17, 2029 | |
| | | |
| | | |
| | | |
| | |
| Timothy Koch | |
| 127,083 | | |
| 116,916 | | |
| 244,000 | | |
$ | 0.15 | | |
February 28, 2028 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 29,166 | | |
| 20,833 | | |
| 50,000 | | |
$ | 1.20 | | |
January 17, 2029 | |
| | | |
| | | |
| | | |
| | |
We have not engaged in any option re-pricings
or other modifications to any of our outstanding equity awards to our Named Executive Officers during fiscal year 2020.
Board of Directors Compensation
Directors who are employees of our
company or of any of our subsidiaries receive no additional compensation for serving on our Board of Directors or any of its committees.
All directors who are not employees of our company or of any of our subsidiaries are compensated at the rate of $9,000 per quarter,
plus $250 per Board meeting attended and are reimbursed for their expenses incurred in attending Board and committee meetings.
The chairmen of our Board committees receive an additional $500 per quarter. On December 1, 2020, each of our non-employee directors
were awarded options to purchase up to 100,000 shares of our common stock.
Code of Ethics
We have adopted a Code of Ethics and Business
Conduct which is applicable to the conduct of our directors, officers and employees, including our CEO, CFO and persons performing
similar functions. A copy of our Code of Business Conduct and Ethics has been filed with the Securities and Exchange Commission
as an exhibit to the Company’s Registration Statement on Form S-1 filed June 28, 2019.
PRINCIPAL STOCKHOLDERS
The following table sets forth, as
of January 22, 2021, the names, addresses and number of shares of our common stock beneficially owned by all persons known to
us to be beneficial owners of more than 5% of the outstanding shares of our common stock, and the names and number of shares beneficially
owned by all of our directors and all of our executive officers and directors as a group (except as indicated, each beneficial
owner listed exercises sole voting power and sole dispositive power over the shares beneficially owned). As of January 11, 2021,
we had a total of 7,649,346 shares of common stock outstanding.
Beneficial ownership is determined in
accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Except as noted
by footnote, and subject to community property laws where applicable, we believe, based on the information provided to us, that
the persons and entities named in the table below have sole voting and investment power with respect to all shares of common stock
shown as beneficially owned by them.
Except as otherwise noted below, the address
for each person or entity listed in the table is c/o Crown Electrokinetics Corp., 1110 NE Circle Blvd., Corvallis, Oregon
97330.
| Name of
Beneficial Owner | |
Number
of
Shares
and Nature
of Beneficial
Ownership(1) | | |
Percent
of
Common
Stock
Outstanding(2) | |
| | |
| | |
| |
| Douglas
Croxall(3) | |
| 4,957,020 | | |
| 45.0 | % |
| | |
| | | |
| | |
| Timothy
Koch(4) | |
| 1,610,348 | | |
| 18.2 | % |
| | |
| | | |
| | |
| Phil
Anderson(5) | |
| 744,643 | | |
| 8.9 | % |
| | |
| | | |
| | |
| DJ
Nag(6) | |
| 33,333 | | |
| 0.4 | % |
| | |
| | | |
| | |
| Edward
Kovalik(6) | |
| 33,333 | | |
| 0.4 | % |
| | |
| | | |
| | |
| John
Marchese(6) | |
| 33,333 | | |
| 0.4 | % |
| | |
| | | |
| | |
| Christopher
Smith(6) | |
| 33,333 | | |
| 0.4 | % |
| | |
| | | |
| | |
| All directors and executive officers as a group
(seven persons) | |
| 7,445,343 | | |
| 73.7 | % |
| (1) |
A person is considered to beneficially own any shares: (i) over which such person, directly or indirectly, exercises sole or shared voting or investment power, or (ii) of which such person has the right to acquire beneficial ownership at any time within 60 days (such as through exercise of stock options or warrants). Unless otherwise indicated, voting and investment power relating to the shares shown in the table for our directors and executive officers is exercised solely by the beneficial owner or shared by the owner and the owner’s spouse or children. |
| (2) |
For purposes of this table, a person or group of persons is deemed to have “beneficial ownership” of any shares of common stock that such person has the right to acquire within 60 days after the date of this prospectus. For purposes of computing the percentage of outstanding shares of our common stock held by each person or group of persons named above, any shares that such person or persons has the right to acquire within 60 days after the date of this prospectus is deemed to be outstanding, but is not deemed to be outstanding for the purpose of computing the percentage ownership of any other person. The inclusion herein of any shares listed as beneficially owned does not constitute an admission of beneficial ownership. |
| (3) |
Includes (i)
526,666 shares of common stock held by Mr. Croxall’s wife, Natasha Allas, (ii) 1,064,682 restricted stock awards and
(iii) options to purchase 3,365,672 shares of common stock. |
| (4)
|
Includes
(i) 400,000 restricted stock awards and (ii) options to purchase 1,210,348 shares of common stock. |
| (5)
|
Reflects
options to purchase 744,643 shares of common stock. |
| (6)
|
Reflects
options to purchase 33,333 shares of common stock. |
From time to time, the number of our shares
held in the “street name” accounts of various securities dealers for the benefit of their clients or in centralized
securities depositories may exceed 5% of the total shares of our common stock outstanding.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Other than compensation arrangements for
our named executive officers and directors, we describe below each transaction or series of similar transactions, since January
1, 2017, to which we were a party or will be a party, in which:
| |
● |
the amounts involved exceeded or will exceed $120,000; and |
| |
● |
any of our directors, executive officers or holders of more than 5% of our capital stock, or any member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest. |
See “Management—Executive
Compensation” for a description of certain arrangements with our executive officers and directors.
DESCRIPTION OF SECURITIES
Our authorized capital stock consists of 200,000,000 shares
of common stock, par value $0.0001 per share, and 50,000,000 shares of preferred stock, par value $0.0001 per share. As of January
11, 2021, 7,649,346 shares of common stock were issued and outstanding and no shares of convertible preferred stock were issued
and outstanding, each such share convertible into one share of common stock. In addition, at such date, 2,620,007 shares of common
stock were reserved for issuance upon the exercise of outstanding common stock purchase warrants.
Common Stock
Voting, Dividend and Other Rights.
Each outstanding share of common stock entitles the holder to one vote on all matters presented to the shareholders for a vote.
Holders of shares of common stock have no cumulative voting, preemptive, subscription or conversion rights. All shares of common
stock to be issued pursuant to this registration statement will be duly authorized, fully paid and non-assessable. Our Board of
Directors determines if and when distributions may be paid out of legally available funds to the holders. To date, we have not
declared any dividends with respect to our common stock. Our declaration of any cash dividends in the future will depend on our
Board of Directors’ determination as to whether, in light of our earnings, financial position, cash requirements and other
relevant factors existing at the time, it appears advisable to do so. We do not anticipate paying cash dividends on the common
stock in the foreseeable future.
Rights Upon Liquidation. Upon liquidation,
subject to the right of any holders of the preferred stock to receive preferential distributions, each outstanding share of common
stock may participate pro rata in the assets remaining after payment of, or adequate provision for, all our known debts and liabilities.
Majority Voting. The holders of
a majority of the outstanding shares of common stock constitute a quorum at any meeting of the shareholders. A plurality of the
votes cast at a meeting of shareholders elects our directors. The common stock does not have cumulative voting rights. Therefore,
the holders of a majority of the outstanding shares of common stock can elect all of our directors. In general, a majority of the
votes cast at a meeting of shareholders must authorize shareholder actions other than the election of directors. Most amendments
to our certificate of incorporation require the vote of the holders of a majority of all outstanding voting shares.
Preferred Stock
Authority of Board of Directors to Create
Series and Fix Rights. Under our certificate of incorporation, as amended, our Board of Directors can issue up to 50,000,000
shares of preferred stock from time to time in one or more series. The Board of Directors is authorized to fix by resolution as
to any series the designation and number of shares of the series, the voting rights, the dividend rights, the redemption price,
the amount payable upon liquidation or dissolution, the conversion rights, and any other designations, preferences or special rights
or restrictions as may be permitted by law. Unless the nature of a particular transaction and the rules of law applicable thereto
require such approval, our Board of Directors has the authority to issue these shares of preferred stock without shareholder approval.
On January 12, 2021, we filed Certificate
of Designation, Preferences and Rights to create our Series A Preferred Stock and Series B Preferred Stock (collectively, “Preferred
Stock”). The preferences, rights and terms of the Series A Preferred Stock and Series B Preferred Stock are identical except
for the conversion price associated with each.
Voluntary Conversion. The Preferred
Stock is convertible at any time at the option of the holder thereof, into that number of shares of common stock determined by
dividing the Stated Value of such Preferred Stock (which is $1,000) by the conversion price. The initial conversion price is $0.4443
for the Series A Preferred Stock and $0.2383 for the Series B Preferred Stock. The initial conversion price shall be adjusted
in the event that we (i) pay a stock dividend or otherwise make a distribution or distributions payable in shares of our common
stock, (ii) subdivide outstanding shares of our common stock into a larger number of shares, (iii) combine (including by way of
a reverse stock split) outstanding shares of our common stock into a small number of shares, or (iv) issue, in the event of a
reclassification of shares of our common stock, any shares of our capital stock.
Mandatory Conversion. If (i)
the closing price of our common stock exceeds 300% of the then-current conversion price for five consecutive trading days, (ii)
the daily average trading volume during thirty consecutive trading days was in excess of $100,000 per trading day, (iii) our common
stock is DWAC eligible and not subject to a “DTC chill” and (iv) the shares of our common stock are freely tradeable
pursuant to Rule 144 of the Securities Act, we have the right to require the holders of Preferred Stock to convert all remaining
shares of Preferred Stock into shares of common stock.
Voting, Dividend and Other Rights.
Holders of Preferred Stock shall have no voting rights. Each outstanding share of Preferred Stock entitles the holder, from and
after the second anniversary of the issuance date thereof, to quarterly dividends at an annual rate of 8% of the Stated Value
per share of Preferred Stock (subject to adjustment), payable in either cash or shares of common stock at our discretion.
Rights Upon Liquidation. In
the event of any liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary, the holders of Preferred
Stock shall be entitled to receive out of our assets an amount equal to the Stated Value for each share of Preferred Stock before
any distribution or payment shall be made to the holders of our common stock. Thereafter, the holders of Preferred Stock shall
be entitled to receive the same amount that a holder of our common stock is entitled to receive if the shares of Preferred Stock
were fully converted into shares of our common stock, which amounts are to be paid pari passu with holders of our common
stock.
Warrants
At September 30, 2020, the following
warrants were outstanding:
| Underlying
Shares of Common Stock |
|
Expiration
Date |
|
Initial
Exercise Price (1) |
| 333,663 |
|
March
31, 2022 |
|
$0.72 |
| 93,035 |
|
May 31, 2022 |
|
$0.72 |
| 54,131 |
|
July 11, 2022
|
|
$0.72 |
| 135,830 |
|
July 27, 2022
|
|
$0.72 |
| 163,960 |
|
August 13, 2022
|
|
$3.39 |
| 55,833 |
|
November 14, 2022
|
|
$3.39 |
| 5,325 |
|
December 24, 2022
|
|
$3.39 |
| 5,322 |
|
December 28, 2022
|
|
$3.39 |
| 47,765 |
|
January 11, 2023
|
|
$3.39 |
| 5,270 |
|
February 15, 2023
|
|
$3.39 |
| 5,263 |
|
February 21, 2023
|
|
$3.39 |
| 10,518 |
|
February 26, 2023
|
|
$3.39 |
| 333,333 |
|
March 2, 2023
|
|
$0.03 |
| 20,998 |
|
March 7, 2023
|
|
$3.39 |
| 5,243 |
|
March 12, 2023
|
|
$3.39 |
| 5,243 |
|
March 13, 2023
|
|
$3.39 |
| 5,242 |
|
March 14, 2023
|
|
$3.39 |
| 5,238 |
|
March 17, 2023
|
|
$3.39 |
| 5,228 |
|
March 27, 2023
|
|
$3.39 |
| 99,230 |
|
April 2, 2023
|
|
$3.39 |
| 4,172 |
|
April 8, 2023
|
|
$3.39 |
| 5,207 |
|
April 16, 2023
|
|
$3.39 |
| 20,602 |
|
June 10, 2023
|
|
$3.39 |
| 115,445 |
|
May 9, 2024 |
|
$1.26 |
| 20,618 |
|
January 10, 2024
|
|
$3.39 |
| 53,455 |
|
March 9, 2024
|
|
$3.39 |
| 53,455 |
|
March 28, 2024
|
|
$3.39 |
| 106,910 |
|
April 29, 2024
|
|
$3.39 |
| 42,763 |
|
May 7, 2024 |
|
$3.39 |
| 10,690 |
|
May 8, 2024 |
|
$3.39 |
| 155,555 |
|
June 1, 2024 |
|
$3.39 |
| 62,222 |
|
June 3 2024 |
|
$3.39 |
| 208,000 |
|
July 7, 2025 |
|
$3.39 |
| 265,267 |
|
September 8, 2025
|
|
$3.75 |
| (1) |
Pursuant to the
terms of such warrants, the exercise price is subject to adjustment in the event of stock splits, combinations or the like
of our common stock. |
Anti-Takeover Effects of Delaware Law
and Our Certificate of Incorporation and Bylaws
Our certificate of incorporation and our
Bylaws contain certain provisions that could have the effect of delaying, deferring or discouraging another party from acquiring
control of us. These provisions and certain provisions of Delaware law, which are summarized below, may discourage coercive takeover
practices and inadequate takeover bids. These provisions also may encourage persons seeking to acquire control of us to first negotiate
with our Board of Directors. We believe that the benefits of increased protection of our potential ability to negotiate with an
unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us because negotiation of these
proposals could result in an improvement of their terms.
Undesignated Preferred Stock. As
discussed above, our Board of Directors has the ability to issue preferred stock with voting or other rights or preferences that
could impede the success of any attempt to change control of us. These and other provisions may have the effect of deferring hostile
takeovers or delaying changes in our control or management.
Delaware Anti-Takeover Statute.
We are subject to the provisions of Section 203 of the Delaware General Corporation Law regulating corporate takeovers. In
general, Section 203 prohibits a publicly held Delaware corporation from engaging, under certain circumstances, in a business
combination with an interested stockholder for a period of three years following the date the person became an interested stockholder
unless:
| |
● |
Prior to the date of the transaction, the Board of Directors of the corporation approved either the business combination or the transaction that resulted in the stockholder’s becoming an interested stockholder; |
| |
|
| |
● |
Upon completion of the transaction that resulted in the stockholder’s becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, but not the outstanding voting stock owned by the interested stockholder, (1) shares owned by persons who are directors and also officers and (2) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| |
|
| |
● |
At or subsequent to the date of the transaction, the business combination is approved by the Board of Directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66-2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
Generally, a business combination includes
a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. An interested
stockholder is a person who, together with affiliates and associates, owns or, within three years prior to the determination of
interested stockholder status, did own 15% or more of a corporation’s outstanding voting stock. We expect the existence of
this provision to have an anti-takeover effect with respect to transactions our Board of Directors does not approve in advance.
We also anticipate that Section 203 may discourage attempts that might result in a premium over the market price for the shares
of common stock held by stockholders.
The provisions of Delaware law and the
provisions of our certificate of incorporation and Bylaws, as amended, could have the effect of discouraging others from attempting
hostile takeovers and, as a consequence, they may also inhibit temporary fluctuations in the market price of our common stock that
often result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing changes
in our management. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders
may otherwise deem to be in their best interests.
Transfer Agent and Registrar
The registrar and transfer agent for our
common stock is Equiniti Trust Company, located at 3200 Cherry Creek South Drive, Suite 430, Denver, Colorado 80209.
SHARES ELIGIBLE FOR FUTURE SALE
Future sales of substantial amounts of
our common stock in the public market, including shares issued upon the exercise of outstanding options or warrants, or upon debt
conversion, or the anticipation of these sales, could adversely affect market prices prevailing from time to time and could impair
our ability to raise capital through sales of equity securities.
Immediately following the closing of
this offering, we will have 9,412,780 shares of common stock issued and outstanding. The common stock sold in this offering will
be freely tradable without restriction or further registration or qualification under the Securities Act.
Previously issued shares of common stock
that were not offered and sold in this offering, as well as shares issuable upon the exercise of warrants and subject to employee
stock options, are or will be upon issuance, “restricted securities,” as that term is defined in Rule 144 under the
Securities Act. These restricted securities are eligible for public sale only if such public resale is registered under the Securities
Act or if the resale qualifies for an exemption from registration under Rule 144 or Rule 701 under the Securities Act, which are
summarized below.
Rule 144
In general, a person who has beneficially
owned restricted shares of our common stock for at least twelve months, or at least six months in the event we have been a reporting
company under the Exchange Act for at least ninety (90) days before the sale, would be entitled to sell such securities, provided
that such person is not deemed to be an affiliate of ours at the time of sale or to have been an affiliate of ours at any time
during the ninety (90) days preceding the sale. A person who is an affiliate of ours at such time would be subject to additional
restrictions, by which such person would be entitled to sell within any three-month period only a number of shares that does not
exceed the greater of the following:
| |
● |
1% of the number of shares of our common stock then outstanding; or |
| |
● |
1% of the average weekly trading volume of our common stock during the four calendar weeks preceding the filing by such person of a notice on Form 144 with respect to the sale; |
provided that, in each case, we are subject
to the periodic reporting requirements of the Exchange Act for at least 90 days before the sale. Rule 144 trades must also comply
with the manner of sale, notice and other provisions of Rule 144, to the extent applicable.
Rule 701
In general, Rule 701 allows a stockholder
who purchased shares of our capital stock pursuant to a written compensatory plan or contract and who is not deemed to have been
an affiliate of ours during the immediately preceding 90 days to sell those shares in reliance upon Rule 144, but without being
required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. All holders
of Rule 701 shares, however, are required to wait until ninety (90) days after the date of this prospectus before selling shares
pursuant to Rule 701.
UNDERWRITING
We have entered into an underwriting
agreement with the several underwriters listed in the table below. Roth Capital Partners, LLC is the representative of the underwriters.
We refer to the several underwriters listed in the table below as the “underwriters.’’
Pursuant to the terms and subject to
the conditions contained in the underwriting agreement, we have agreed to sell to the underwriters named below, and each underwriter
severally has agreed to purchase from us, the respective number of shares of common stock set forth opposite its name below:
| Underwriter | |
Number of
Shares | |
| Roth Capital Partners, LLC | |
| |
| National Securities Corporation | |
| |
| Colliers Securities LLC | |
| |
| Total | |
| 1,030,303 | |
The underwriting agreement provides
that the obligation of the underwriters to purchase the shares of common stock offered by this prospectus is subject to certain
conditions. The underwriters are obligated to purchase all of the shares of common stock if any such shares of common stock are
purchased. However, the underwriters are not required to purchase the shares of common stock covered by the underwriters’
option described below.
We have granted the underwriters an
option, exercisable for 30 days from the date of this prospectus, to purchase up to an aggregate of additional shares of
common stock at the public offering price per share, less underwriting discounts and commissions.
Discounts, Commissions and Expenses
The underwriters propose to offer the
shares of common stock purchased pursuant to the underwriting agreement to the public at the combined public offering price set
forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per
share. After this offering, the combined public offering price and concession may be changed by the underwriters. No such change
shall change the amount of proceeds to be received by us as set forth on the cover page of this prospectus.
In connection with the sale of the
common stock to be purchased by the underwriters, the underwriters will be deemed to have received compensation in the form of
underwriting commissions and discounts. The underwriters’ commissions and discounts will be 8% of the gross proceeds of
this offering, or $ per share of common stock, based on
the public offering price set forth on the cover page of this prospectus.
We have also agreed to reimburse Roth
Capital Partners at closing for out-of-pocket expenses incurred by it in connection with the offering up to a maximum of $135,000.
The following table shows the underwriting
discounts and commissions payable to the underwriters by us in connection with this offering (assuming both the exercise and non-exercise
of the over-allotment option to purchase additional shares of common stock we have granted to the underwriters):
| | |
Per
Share | | |
Total | |
| | |
Without
Over- allotment | | |
With
Over- allotment | | |
Without
Over- allotment | | |
With
Over- allotment | |
| Public offering price | |
$ | | | |
$ | | | |
| | | |
| | |
| Underwriting discounts and commissions paid by
us | |
$ | | | |
$ | | | |
|
| | |
| | |
Underwriter Warrants
Upon the closing of this offering, we have agreed to issue
to the underwriters warrants, or the underwriter warrants, to purchase a number of shares of common stock equal to up to 8% of
the total shares of common stock sold in the initial closing of this public offering. The underwriter warrants will be exercisable
at a per share exercise price equal to 125% of the public offering price per share of common stock sold in this offering. The
underwriter warrants are exercisable at any time and from time to time, in whole or in part, during the four and a half year period
commencing six months after the effective date of the registration statement related to this offering.
The underwriter warrants and the shares
of common stock underlying the underwriter warrants have been deemed compensation by the Financial Industry Regulatory Authority,
or FINRA, and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The underwriter, or permitted
assignees under such rule, may not sell, transfer, assign, pledge, or hypothecate the underwriter warrants or the securities underlying
the underwriter warrants, nor will the underwriter engage in any hedging, short sale, derivative, put, or call transaction that
would result in the effective economic disposition of the underwriter warrants or the underlying shares for a period of 180 days
from the effective date of the registration statement. Additionally, the underwriter warrants may not be sold, transferred, assigned,
pledged or hypothecated for a 180-day period following the effective date of the registration statement except to any underwriter
and selected dealer participating in this offering and their bona fide officers or partners. The underwriter warrants will provide
for adjustment in the number and price of the underwriter warrants and the shares of common stock underlying such underwriter
warrants in the event of recapitalization, merger, stock split or other structural transaction, or a future financing undertaken
by us.
Nasdaq Listing
We are in the process of applying to
have our shares of common stock listed on Nasdaq under the symbol “CRKN”. There is no assurance that a market will
develop for our common stock.
Indemnification
Pursuant to the underwriting agreement,
we have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to
contribute to payments that the underwriters or such other indemnified parties may be required to make in respect of those liabilities.
Lock-Up Agreements
We have agreed not to (i) offer,
pledge, issue, sell, contract to sell, purchase, contract to purchase, lend or otherwise transfer or dispose of, directly or indirectly,
any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock; (ii) enter
into any swap or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of shares
of common stock; or (iii) file any registration statement with the Commission relating to the offering of any shares of our
common stock or any securities convertible into or exercisable or exchangeable for shares of our common stock, without the prior
written consent of Roth Capital Partners for a period of 90 days following the date of this prospectus(the “Lock-up
Period”). These restrictions on future issuances are subject to exceptions for (i) the issuance of the securities
sold in this prospectus, (ii) the issuance of common stock upon the exercise of options or warrants or the conversion of outstanding
preferred stock or other outstanding convertible securities disclosed as outstanding in the Registration Statement (excluding
exhibits thereto), the Time of Sale Disclosure Package, and the Final Prospectus, and (iii) the issuance of employee stock options
not exercisable during the Lock-Up Period and the grant of restricted stock awards or restricted stock units or shares of common
stock pursuant to equity incentive plans described in this prospectus.
In addition, each of our directors
and executive officers has entered into a lock-up agreement with the underwriters. Under the lock-up agreements, the directors
and executive officers may not, directly or indirectly, sell, offer to sell, contract to sell, or grant any option for the sale
(including any short sale), grant any security interest in, pledge, hypothecate, hedge, establish an open “put equivalent
position” (within the meaning of Rule 16a-1(h) under the Securities Exchange Act of 1934, as amended, or the Exchange
Act), or otherwise dispose of, or enter into any transaction which is designed to or could be expected to result in the disposition
of, any shares of our common stock or securities convertible into or exchangeable for shares of our common stock, or publicly
announce any intention to do any of the foregoing, without the prior written consent of Roth Capital Partners, for a period of
90 days from the closing date of this offering.. These restrictions on future dispositions by our directors and executive
officers are subject to exceptions including for (i) transfers or dispositions as a bona fide gift or gifts, or for bona fide
estate planning purposes, (ii) transfers to immediate family, and (iii) transfers as a bona fide gift to a charity or educational
institution.
Electronic Distribution
This prospectus may be made available
in electronic format on websites or through other online services maintained by the underwriters or by their affiliates. In those
cases, prospective investors may view offering terms online and prospective investors may be allowed to place orders online. Other
than this prospectus in electronic format, the information on the underwriters’ websites or our website and any information
contained in any other websites maintained by the underwriters or by us is not part of this prospectus or the registration statement
of which this prospectus forms a part, has not been approved and/or endorsed by us or the underwriter in its capacity as
underwriter, and should not be relied upon by investors.
Price Stabilization, Short Positions
and Penalty Bids
In connection with the offering the
underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions and penalty
bids in accordance with Regulation M under the Exchange Act:
| ● | Stabilizing
transactions permit bids to purchase the underlying security so long as the stabilizing
bids do not exceed a specified maximum. |
| | | |
| ● | Over-allotment
involves sales by the underwriters of shares in excess of the number of shares the underwriters
are obligated to purchase, which creates a syndicate short position. The short position
may be either a covered short position or a naked short position. In a covered short
position, the number of shares over-allotted by the underwriters is not greater than
the number of shares that they may purchase in the over-allotment option. In a naked
short position, the number of shares involved is greater than the number of shares in
the over-allotment option. The underwriters may close out any covered short position
by either exercising their over-allotment option and/or purchasing shares in the open
market. |
| | | |
| ● | Syndicate
covering transactions involve purchases of the common stock in the open market after
the distribution has been completed in order to cover syndicate short positions. In determining
the source of shares to close out the short position, the underwriters will consider,
among other things, the price of shares available for purchase in the open market as
compared to the price at which they may purchase shares through the over-allotment option.
A naked short position occurs if the underwriters sell more shares than could be covered
by the over-allotment option. This position can only be closed out by buying shares in
the open market. A naked short position is more likely to be created if the underwriters
are concerned that there could be downward pressure on the price of the shares in the
open market after pricing that could adversely affect investors who purchase in the offering. |
| ● | Penalty bids permit the
underwriters to reclaim a selling concession from a syndicate member when the common
stock originally sold by the syndicate member is purchased in a stabilizing or syndicate
covering transaction to cover syndicate short positions. |
These stabilizing transactions, syndicate
covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing
or retarding a decline in the market price of the common stock. As a result, the price of our common stock may be higher than
the price that might otherwise exist in the open market. These transactions may be discontinued at any time.
Neither we nor the underwriters make
any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have
on the price of our shares of common stock. In addition, neither we nor the underwriters make any representation that the underwriter
will engage in these transactions or that any transaction, if commenced, will not be discontinued without notice.
Offer restrictions outside the United
States
Other than in the United States, no
action has been taken by us or the underwriter that would permit a public offering of the securities offered by this prospectus
in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or
sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the
offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result
in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes
are advised to inform themselves about and to observe any restrictions relating to this offering and the distribution of this
prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by
this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure
document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments
Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian
Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is
lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions
set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons
as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this
offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under
the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree
within 12 months after its transfer to the offeree under this prospectus.
Canada
The securities may be sold in Canada
only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National
Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined
in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities
must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable
securities laws. Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for
rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies
for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the
purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation
of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor. Pursuant to section
3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriter is not required to comply with the disclosure
requirements of NI33-105 regarding underwriter conflicts of interest in connection with this offering.
China
The information in this document does
not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China
(excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan).
The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to
“qualified domestic institutional investors.”
European Economic Area — Belgium,
Germany, Luxembourg and Netherlands
The information in this document has
been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC
(“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member
State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of securities
has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the
Prospectus Directive as implemented in that Relevant Member State:
| ● | to
legal entities that are authorized or regulated to operate in the financial markets or,
if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
| ● | to
any legal entity that has two or more of (i) an average of at least 250 employees
during its last fiscal year; (ii) a total balance sheet of more than €43,000,000
(as shown on its last annual unconsolidated or consolidated financial statements) and
(iii) an annual net turnover of more than €50,000,000 (as shown on its last annual
unconsolidated or consolidated financial statements); |
| ● | to
fewer than 100 natural or legal persons (other than qualified investors within the meaning
of Article 2(1)(e) of the Prospectus Directive) subject to obtaining our prior consent
or any underwriter for any such offer; or |
| ● | in
any other circumstances falling within Article 3(2) of the Prospectus Directive,
provided that no such offer of securities shall require us to publish a prospectus pursuant
to Article 3 of the Prospectus Directive. |
France
This document is not being distributed
in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning
of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq.
of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have
not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering
material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly,
may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions
have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own
account, as defined in and in accordance with Articles L.411-2-II-2 and D.411-1 to D.411-3, D. 744-1, D.754-1 and D.764-1 of the
French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors
(cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2°
and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the
General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly)
to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of
the French Monetary and Financial Code.
Ireland
The information in this document does
not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish
regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within
the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The
securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way
of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations
and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
The securities offered by this prospectus
have not been approved or disapproved by the Israeli Securities Authority (the ISA), or ISA, nor have such securities been registered
for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication
of a prospectus. The ISA has not issued permits, approvals or licenses in connection with this offering or publishing the prospectus;
nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to
the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered
by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities
laws and regulations.
Italy
The offering of the securities in the
Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Società
e la Borsa, “CONSOB” pursuant to the Italian securities legislation and, accordingly, no offering material relating
to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within
the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other
than:
| ● | to
Italian qualified investors, as defined in Article 100 of Decree no. 58 by reference
to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation
no. 1197l”) as amended (“Qualified Investors”); and |
| ● | in
other circumstances that are exempt from the rules on public offer pursuant to Article 100
of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
Any offer, sale or delivery of the
securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor
solicits an offer from the issuer) under the paragraphs above must be:
| ● | made
by investment firms, banks or financial intermediaries permitted to conduct such activities
in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended),
Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other
applicable laws; and |
| ● | in
compliance with all relevant Italian securities, tax and exchange controls and any other
applicable laws. |
Any subsequent distribution of the
securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No.
58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules
may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities
for any damages suffered by the investors.
Japan
The securities have not been and will
not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948),
as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement
of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL
and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in
Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional
Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and
acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
New Zealand
The shares of common stock offered
hereby have not been offered or sold, and will not be offered or sold, directly or indirectly in New Zealand and no offering materials
or advertisements have been or will be distributed in relation to any offer of shares in New Zealand, in each case other than:
| ● | to
persons whose principal business is the investment of money or who, in the course of
and for the purposes of their business, habitually invest money; |
| | | |
| ● | to
persons who in all the circumstances can properly be regarded as having been selected
otherwise than as members of the public; |
| | | |
| ● | to
persons who are each required to pay a minimum subscription price of at least NZ$500,000
for the shares before the allotment of those shares (disregarding any amounts payable,
or paid, out of money lent by the issuer or any associated person of the issuer); or |
| | | |
| ● | in
other circumstances where there is no contravention of the Securities Act 1978 of New
Zealand (or any statutory modification or reenactment of, or statutory substitution for,
the Securities Act 1978 of New Zealand). |
Portugal
This document is not being distributed
in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within
the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities
have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document
and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities
Market Commission (Comissăo do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not
be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that
are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities
in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only
such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will
not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document
may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed
not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella
instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined
in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the
information contained in it to any other person.
Switzerland
The securities may not be publicly
offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or
regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance
prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses
under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland.
Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made
publicly available in Switzerland.
Neither this document nor any other
offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In
particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market
Supervisory Authority (FINMA).
This document is personal to the recipient
only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities
have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental
authority in the United Arab Emirates, nor have we received authorization or licensing from the Central Bank of the United Arab
Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab
Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. We may not render services
relating to the securities within the United Arab Emirates, including the receipt of applications and/or the allotment or redemption
of such shares.
No offer or invitation to subscribe
for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document
nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United
Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”))
has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis
to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may
not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except
in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not
be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person
in the United Kingdom.
Any invitation or inducement to engage
in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities
has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United
Kingdom in circumstances in which section 21(1) of FSMA does not apply us.
In the United Kingdom, this document
is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments
falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions)
Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high
net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated
(together “relevant persons”). The investments to which this document relates are available only to, and any invitation,
offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should
not act or rely on this document or any of its contents.
LEGAL MATTERS
Certain legal matters in connection
with the offering and the legality of the issuance of the shares offered in this prospectus will be passed upon for us by Pryor
Cashman LLP, New York, New York. Certain legal matters in connection with the offering was passed upon for the underwriter by
Sichenzia Ross Ference LLP, New York, New York.
EXPERTS
Marcum LLP, an independent registered public
accounting firm, has audited our financial statements at March 31, 2020 and 2019 and for the years ended March 31, 2020 and 2019
as set forth in its report included in our annual report on Form 10-K for the year ended March 31, 2020, which is incorporated
by reference into this prospectus and elsewhere in the registration statement of which this prospectus is a part. Our financial
statements are incorporated by reference in reliance on Marcum LLP’s reports, given on their authority as experts in accounting
and auditing.
INFORMATION INCORPORATED BY REFERENCE
The Securities and Exchange Commission
allows us to “incorporate by reference” information that we file with it into this prospectus, which means that we
can disclose important information to you by referring you to those documents. The information incorporated by reference is an
important part of this prospectus. The information incorporated by reference is considered to be a part of this prospectus, and
information that we file later with the SEC will automatically update and supersede information contained in this prospectus.
We incorporate by reference the documents
listed below that we have previously filed with the Securities and Exchange Commission:
| |
● |
our Annual Report
on Form 10-K
for the fiscal year ended March 31, 2020, filed with the Securities and Exchange Commission on September 4, 2020; |
| |
|
|
| |
● |
our Quarterly
Reports on Form 10-Q for the periods ended June 30, 2020
and September 30, 2020,
filed with the Securities and Exchange Commission on October 6, 2020 and November 17, 2020, respectively; and |
| |
|
|
| |
● |
our Current Reports
on Form 8-K filed with the Securities and Exchange Commission on September 14, 2020,
November 18, 2020,
December 7, 2020
and January 21, 2021
(other than information “furnished” under Items 2.02 or 7.01, or corresponding information furnished under Item
9.01 or included as an exhibit). |
All reports and other documents that
we file with the Securities and Exchange Commission under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after
the date of this prospectus but prior to the termination of the offering of the shares hereunder will also be considered to be
incorporated by reference into this prospectus from the date of the filing of these reports and documents, and will supersede
the information herein; provided, however, that all reports, exhibits and other information that we “furnish”
to the Securities and Exchange Commission will not be considered incorporated by reference into this prospectus. Any statement
contained in a document incorporated by reference in this prospectus shall be deemed to be modified or superseded to the extent
that a statement contained herein, therein or in any other subsequently filed document that also is incorporated by reference
herein or therein modifies or supersedes such statement. Any statement so modified or superseded shall not be deemed, except as
so modified or superseded, to constitute a part of this prospectus or the registration statement.
We will provide you without charge, upon
your oral or written request, with a copy of any or all reports, proxy statements and other documents we file with the Securities
and Exchange Commission, as well as any or all of the documents incorporated by reference in this prospectus or the registration
statement (other than exhibits to such documents unless such exhibits are specifically incorporated by reference into such documents).
Requests for such copies should be directed to Crown Electrokinetics Corp., Attn: Chief Financial Officer, 1110 NE Circle Blvd.,
Corvallis, Oregon 97330. You may also direct any requests for documents to us by telephone at (800) 674-3612.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the Securities and Exchange
Commission a registration statement on Form S-1 (including exhibits) under the Securities Act, with respect to the shares to be
sold in this offering. This prospectus does not contain all the information set forth in the registration statement. For further
information with respect to our company and the common stock offered in this prospectus, reference is made to the registration
statement, including the exhibits filed thereto, and the financial statements and notes filed as a part thereof. With respect to
each such document filed with the Securities and Exchange Commission as an exhibit to the registration statement, reference is
made to the exhibit for a more complete description of the matter involved.
We file quarterly and annual reports, proxy
statements and other information with the Securities and Exchange Commission. You may read and copy any document that we file at
the public reference facilities of the Securities and Exchange Commission in Washington, D.C. The Securities and Exchange Commission
maintains a website that contains reports, proxy and other information statements about issuers, including us, that file electronically
with the Securities and Exchange Commission. The address of the website is http://www.sec.gov.
No dealer, salesperson, or other person
has been authorized to give any information or to make any representation not contained in this prospectus, and, if given or made,
such information and representation should not be relied upon as having been authorized by us or the selling stockholder. This
prospectus does not constitute an offer to sell or a solicitation of an offer to buy any of the shares offered by this prospectus
in any jurisdiction or to any person to whom it is unlawful to make such offer or solicitation. Neither the delivery of this prospectus
nor any sale made hereunder shall under any circumstances create an implication that there has been no change in the facts set
forth in this prospectus or in our affairs since the date hereof.
Until ,
all dealers that effect transactions in these shares, whether or not participating in this offering, may be required to deliver
a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with
respect to their unsold overallotments or subscriptions.
Crown
ElectroKinetics Corp.
1,030,303 SHARES OF COMMON STOCK
PROSPECTUS
,
2021
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance
and Distribution
The following table sets forth all
expenses to be paid by the registrant, other than estimated underwriting discounts and commissions, in connection with this offering.
All amounts shown are estimates except for the SEC registration fee and the FINRA filing fee:
| SEC registration fee | |
$ | 2,040.17 | |
| Nasdaq listing fee | |
$ | 45,000 | |
| FINRA filing fee | |
$ | 3,050 | |
| Printing expenses | |
$ | 5,000 | |
| Legal fees and expenses | |
$ | 50,000 | |
| Accounting fees and expenses | |
$ | 25,000 | |
| Miscellaneous | |
$ | 4,909.83 | |
| Total | |
$ | 135,000 | |
Item 14. Indemnification of Directors
and Officers
Under Delaware law, a Delaware corporation
may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action,
suit or proceeding, whether civil, criminal, administrative or investigative (other than one by or in the right of the corporation)
by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving
at the request of the corporation as a director, officer, employee or agent of another corporation, against judgments, fines, amounts
paid in settlement and reasonable expenses, including attorneys’ fees actually and necessarily incurred as a result of such
action or proceeding, if such director or officer acted, in good faith, for a purpose which such person reasonably believed to
be, in, or not opposed to, the best interests of the corporation and, in criminal actions or proceedings, in addition, had no reasonable
cause to believe that such conduct was unlawful.
In the case of a derivative action, a Delaware
corporation may indemnify any such person against expense, including attorneys’ fees actually and necessarily incurred by
such person in connection with the defense or settlement of such action or suit if such director or officer if such director or
officer acted, in good faith, for a purpose which such person reasonably believed to be, in or not opposed to, the best interests
of the corporation, except that no indemnification will be made in respect on any claim, issue or matter as to which such person
will have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery of the State of
Delaware or any other court in which such action was brought determines such person is fairly and reasonably entitled to indemnity
for such expense.
Delaware Law permits a corporation to include
in its certificate of incorporation a provision eliminating or limiting a director’s liability to a corporation or its stockholders
for monetary damages for breaches of fiduciary duty. Delaware Law provides, however, that liability for breaches of the duty of
loyalty, acts or omissions not in good faith or involving intentional misconduct, or knowing violation of the law, and the unlawful
purchase or redemption of stock or payment of unlawful purchase or redemption of stock or payment of unlawful dividends or the
receipt of improper personal benefits cannot be eliminated or limited in this manner.
Our Certificate of Incorporation and Bylaws
provide that we will indemnify our directors to the fullest extent permitted by Delaware law and may, if and to the extent authorized
by the Board of Directors, indemnify our officers and any other person whom we have the power to indemnify against any liability,
reasonable expense or other matter whatsoever.
Any amendment, modification or repeal of
the foregoing provisions shall be prospective only, and shall not affect any rights or protections of any of our directors existing
as of the time of such amendment, modification or repeal.
We may also, at the discretion of the Board
of Directors, purchase and maintain insurance to the fullest extent permitted by Delaware law on behalf of any of our directors,
officers, employees or agents against any liability asserted against such person and incurred by such person in any such capacity.
Insofar as indemnification for liabilities
arising under the Securities Act may be permitted to directors, officers or persons controlling the Registrant pursuant to the
foregoing, the Registrant has been informed that in the opinion of the SEC such indemnification is against public policy as expressed
in the Securities Act and is therefore unenforceable.
Item 15. Recent Sales of Unregistered
Securities
The information contained in “Notes
to Financial Statements - Note 8 – Notes Payable,” “Notes to Financial Statements - Note 9 – Stockholders’
Equity” and “Notes to Financial Statements - Note 10 – Stock-based Compensation, Restricted Stock
and Stock Options” contained in the notes to the financial statements incorporated by reference herein is incorporated
by reference herein.
On September 11, 2020, Crown entered
into a Securities Purchase Agreement with certain institutional and accredited investors to sell to such investors an aggregate
of 463,333 unregistered shares of common stock and 231,666 warrants to purchase common stock for gross proceeds of approximately
$1,737,500. The shares of common stock were issued at a price of $3.75 per share. The Special Equities Group, a division of Bradley
Woods & Co. Ltd., acted as exclusive placement agent for the transaction and received cash compensation of $126,000 and 33,600
warrants to purchase shares of common stock.
On November 13, 2020, Crown entered
into a Securities Purchase Agreement with certain institutional and accredited investors to sell to the investors senior convertible
debentures, convertible into Crown’s common stock at a conversion price of $3.75 per share, subject to adjustment as set
forth in the debenture. The debentures have a maturity of one year, accrue interest at the rate of 7% per year, and are subject
to 12.5% original issue discount. Each investor also received a warrant to purchase a number of share of Crown’s common
stock equal to 50% of the number of shares the debenture is convertible into. The warrants have a five year term, and an exercise
price of $4.65 per share, subject to adjustment as set forth therein.
The above mentioned securities were issued
in reliance on the exemption from registration provided by Section 4(a)(2) of the Securities Act and/or Rule 506 of Regulation
D promulgated thereunder.
Item 16. – Exhibits and Financial
Statement Schedules.
(b) Exhibits:
| 1.1 |
Form
of Underwriting Agreement* |
| |
|
| 3.1 |
Certificate
of Incorporation of the Registrant filed April 20, 2015 with the Delaware Secretary of State (and amendments thereto).* |
| |
|
| 3.2 |
By-laws
of the Registrant.* |
| |
|
| 3.3 |
Form of Amended and Restated Certificate of Designations, Preferences and Rights of Series A Preferred Stock of Crown Electrokinetics Corp.*** |
| |
|
| 3.4 |
Form of Amended and Restated Certificate of Designations, Preferences and Rights of Series B Preferred Stock of Crown Electrokinetics Corp.*** |
| |
|
| 4.1 |
Form
of Underwriter Warrant* |
| |
|
| 5.1 |
Opinion of Pryor Cashman LLP, regarding legality of shares being registered.*** |
| |
|
| 10.1 |
Intellectual
Property Agreement, dated as of January 31, 2016, between Hewlett-Packard Development Company, L.P. and 3D Nanocolor Corp.
(and amendments thereto).* |
| |
|
| 10.2 |
Collaboration
Agreement, dated as of August 23, 2017, between 3D Nanocolor Corp. and Eastman Chemical Company (and amendment thereto).* |
| |
|
| 10.3 |
Agreement,
dated as of November 15, 2017, between Crown Electrokinetics Corp. and Asahi Glass Co., Ltd. (and amendment thereto).* |
| |
|
| 10.4 |
Agreement,
dated as of February 1, 2019, between Crown Electrokinetics Corp. and AGC Inc. (f/k/a Asahi Glass Co., Ltd.).* |
| |
|
| 14.1 |
Code
of Business Conduct and Ethics of the Registrant.* |
| |
|
| 21.1 |
List
of Subsidiaries of Registrant.* |
| |
|
| 23.1 |
Consent of Marcum LLP.*** |
| |
|
| 23.2 |
Consent of Pryor Cashman LLP (included in their opinion filed as Exhibit 5.1).*** |
| |
|
| 24.1 |
Powers
of Attorney* |
| * |
Previously filed. |
| ** |
To be filed by amendment. |
| *** |
Filed herewith. |
Item 17. Undertakings
The undersigned registrant hereby undertakes:
| (1) |
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| |
(i) |
to include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| |
(ii) |
to reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| |
(iii) |
to include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| (2) |
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) |
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (4) |
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| |
(i) |
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| |
(ii) |
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| |
(iii) |
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| |
(iv) |
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| (5) |
The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (6) |
For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective. |
| (7) |
For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
SIGNATURES
In accordance with the requirements
of the Securities Act of 1933, the Registrant certifies that it has reasonable grounds to believe that it met all the requirements
of filing on Form S-1 and authorized this Amendment No. 3 to the Registration Statement to be signed on its behalf by the undersigned,
in Corvallis, Oregon, on January 25, 2021.
| |
Crown Electrokinetics Corp. |
| |
|
| |
By: |
/s/ Douglas Croxall |
| |
|
Douglas Croxall |
| |
|
Chief Executive Officer |
Pursuant to the requirements of the
Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates
indicated.
| /s/
Douglas Croxall |
|
Dated:
January 25, 2021 |
Name: Douglas Croxall
Title: Chief Executive Officer (Principal Executive
Officer) |
|
|
| |
|
|
| /s/
Phil Anderson |
|
Dated: January
25, 2021 |
Name: Phil Anderson
Title: Chief Financial Officer (Principal
Financial Officer and Principal Accounting
Officer) |
|
|
| |
|
|
| * |
|
Dated: January
25, 2021 |
| Name: Dr. DJ Nag |
|
|
| Title: Director |
|
|
| |
|
|
| /s/
Edward Kovalik |
|
Dated: January
25, 2021 |
| Name: Edward Kovalik |
|
|
| Title: Director |
|
|
| |
|
|
| /s/
John Marchese |
|
Dated: January
25, 2021 |
| Name: John Marchese |
|
|
| Title: Director |
|
|
| |
|
|
| /s/
Christopher Smith |
|
Dated: January
25, 2021 |
| Name: Christopher Smith |
|
|
| Title: Director |
|
|
| * | Pursuant to power of attorney |
| By: |
/s/
Douglas Croxall |
|
|
| |
Douglas Croxall |
|
|
| |
Attorney-in-Fact |
|
|
II-4







